10:27 Revised ED - aos-hci-2012-research
advertisement

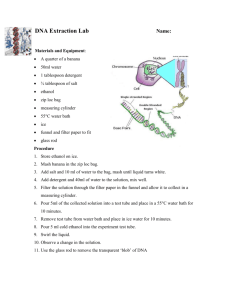
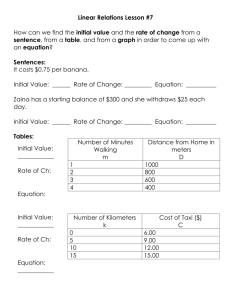
Aman Mangalmurti Kara Newman October 27, 2011 Filtration and Ethanol Yield From Banana Peels of Varying Age Problem Can an optimal ripeness of bananas be found which will allow for maximum ethanol production and filtration ability? This is important to find because banana peels are usually a waste product; many plantations have been ravaged by disease, but their peels could still be used as a filter for heavy metals and converted to bio-ethanol. Hypothesis 1. If ripeness increases, then filtration ability decreases. This is because cellulose breaks down into sugar; filtration ability increases with larger amounts of cellulose. 2. If ripeness increases, then ethanol yield increases. The sugar content of banana peels increases with age, and ethanol yield increases with more sugar. 3. If banana peels are used for filtration, then ethanol yield will not change because the chemical composition remains unchanged. Independent Variable1. Ripeness of Bananas 2. Ripeness of Bananas 3. Filtration Dependent Variable 1. Filtration Ability 2. Ethanol Production 3. Ethanol Production Constants fruit (bananas), concentration of metals in the solution being filtered/tested, pretreatment method, method of filtration, method of ethanol production (SSF) Control - 1) Water will be run through the banana peel filters to test for metals originating in the banana 2) Amount of ethanol produced without a filter (to be compared with ethanol production after filtration) Repeated Trials 150 bananas will be tested. 75 of the bananas will be tested first with filtration, and 75 will be tested only for ethanol production. Materials Approximately 150 pounds of bananas (Yeast) Saccharyomyces cerevisiae Lead (II) Sulfate 150 mL Erlenmeyer flasks with stoppers Hot Plate Distilled Water MgSO4∙7H2O 0.1 and (magnesium sulfide hydrate) KH2PO4 0.1 (potassium phosphate) Sterilized 5N sodium hydroxide solution PVDF membrane Digital multimeter Blender or food processor Distillation apparatus Peptone Butter knife Centrifuge Yeast malt extract broth Incubator Quincy Lab Model 30 GC hot-air oven Procedure Using Banana Peels For Filtration 1. Remove banana peels from the fruit. 2. Insert both probes of the multimeter into the flesh of the banana, 3 inches apart, ensuring that it is in the correct mode to measure resistance. Take three readings. 3. Dry banana peels through dehydration[1], (put the banana peels in the hot air oven and dry them at 60 degrees for 23 hours). 4. Using a rotary mill to grind dehydrated banana peels. 5. Sieve to 1 mm particle size. 6. Create 1 M lead solution by introducing 331g Pb(NO3)2 into 1 L distilled water. 7. Introduce the 1mm banana peel particles into solution of heavy metals. 8. Remove banana product. 9. Using a lead reagent the remaining concentration of lead ions will be found Procedure Cont. (Ethanol Production) Preparation of Yeast Cells 1. Add 50ml sterlized yeast malt extract into ten 150ml Erlenmeyer flasks 2. The dried yeast powder (S. cerevisiae) was aseptically inoculated into ten 150-ml Erlenmeyer flasks each containing 50 ml sterilized yeast malt extract (YM) broth. 3. The flasks were incubated at 30 °C for 48 h and 100 rpm in an incubator shaker (Innova 4000, New Brins-wick Scientific, USA). 4. Ten percent of the inoculum was aseptically transferred to each of 10 sterilized 250-ml Erlenmeyer flasks containing 100 ml sterilized PYGAL medium composed of (%; w/v) pep-tone 0.3, yeast extract 0.3, KH2PO4 0.1, MgSO4∙7H2O 0.1 and galactose 2.0. 5. The pH of the medium was adjusted to 5.6 and the flasks were incubated at 35 °C for 24 h at 100 rpm in an incubator shaker. 6. Fifty milliliters of the inoculum obtained from each flask after 24 h incubation was aseptically transferred to a 1-l flask containing 500 ml sterilized PYGAL medium. 7. The 10 1-l flasks were incubated at 35 °C for 24 h at 100 rpm in an incubator shaker. PYGAL medium was prepared fresh each time, just prior to the fermentation experiments. 8. Cells were concentrated by centrifugation of culture in sterilized centrifuge tubes at 10,000 g at 4 °C for 10 min in a refrigerated centrifuge (Sorvall superspeed FCC B, Sorvall Inc., USA). Pretreatment and SSF 9. Peel bananas. 10. Insert both probes of the multimeter into the flesh of the banana, 3 inches apart, ensuring that it is in the correct mode to measure resistance. Take three readings. 11. Dehydrate the banana peels using the Quincy Lab Model 30 GC Hot-air oven1 (put the banana peels in the hot air oven and dry them at 60 degrees for 23 hours.) 12. Using a rotary mill or food processor, grind the banana peels into small pieces and then sieve banana ground banana peels to a particle size of 1 mm or smaller. 13. Suspend dried and ground banana peel powder in distilled water at a solid-to-liquid loading of 10% (w/v) in baffled polycarbonate capped flasks along with 20% w/v yeast extract, 20% w/v peptone, and 10% w/v MgSO4. (Pretreatment Stage) 14. Subject the banana powder mixture to an autoclave-sterilization process at 121 °C, 15 psi for 15 minutes. 15. Cool the mixture by placing the erlen-meyer flasks in a bath of cold water. 16. Adjust the pH of the mixture to 5.5 using sterilized 5N sodium hydroxide solution 17. Add 7.5 mL of yeast (S. cerevisiae) (yeast cells produced in steps 5-12) 18. Filter-sterilize the enzymes (ϐ-glucosidase) using 0.45 чm PVDF membranes and add them to the mixture. It will take about 20 hours for the mixture to ferment 19. Distill the fermented solution 20. Record the amount of ethanol produced in (mL of ethanol/g of banana powder) References Oberoi, H.S., Vadlani, P.V., Saida, L., Bansal, S., & Hughes, J.D. (2011). Ethanol production from banana peels using statistically optimized simultaneous saccharification and fermentation process. Waste Management, 31(7), 1576-1584. doi:10.1016/j.wasman.2011.02.007 Castro, R. Caetano, L. Ferreira, G. Padilha, P. Saeki, M. Zara, L. Martines, M. Castro, G. (2011). Banana Peel Applied to the Solid Phase Extraction of Copper and Lead from River Water: Preconcentration of Metal Ions with a Fruit Waste. Industrial & Engineering Chemistry Research. Retrieved June 6, 2011, from http://pubs.acs.org/doi/abs/10.1021/ie101499e Quincy Lab, Inc. (2008). Model GC Series Lab Ovens: OPERATING MANUAL. Retrieved October 2, 2011 from <http://affordablelabovens.com/docs/pdfs/10GC.pdf> ScienceLab.com, Inc. (2005, October 10). Ethanol Material Safety Data Sheet. Retrieved October 2, 2011, from: http://www.sciencelab.com/xMSDS 2_2_Methoxyethoxy_ethanol9924648 ScienceLab.com, Inc. (2005, October 10). Magnesium Sulfate Material Safety Data Sheet. Retrieved October 2, 2011, from: http://www.sciencelab.com/msds.php?msdsId=9927218 ScienceLab.com, Inc. (2005, October 10). Peptone Material Data Safety Sheet. Retrieved October 2, 2011 from: http://www.sciencelab.com/msds.php?msdsId=9927386 ScienceLab.com, Inc. (2005, October 10). Lead (II) Nitrate Material Data Safety Sheet. Retrieved October 2, 2011, from :http://www.sciencelab.com/msds.php?msdsId=9924473 [1] 1: Remove the glass thermometer from its container and place bottom end through the rubber grommet. Insert grommet into one of the ports on the top of the oven and adjust the thermometer so that it extends at least 2 inches into the oven chamber. Switch the power switch to the ON position. Turn the thermostat control knob clockwise to around the number '8'. Monitor the reading of the thermometer until it reaches the desired temperature. Slowly rotate the control knob counter-clockwise until the heat-cycle light goes out. Turn the oven off with the ON/OFF power switch. Switch on the ON/OFF power switch. Turn the thermostat knob to the desired temperature indicated on the knob (Fahrenheit), or the dial (Centigrade). Rotate the thermostat dial to the desired temperature. The heat cycle light will illuminate until the set temperature is reached. Once reached, the heat-cycle light will cycle on and off with the heaters maintaining set temperature. Typically, the oven needs to cycle at a set temperature for at least 20 minutes before it will achieve equilibrium and become temperature stable. Statistics 1. What are you measuring and how are going to measure it? We are measuring 1) filtration ability (mol/gram) 2) ethanol yield (mL ethanol/g banana peel) 2. What are you comparing? a) The filtration ability (mol/gram), at different banana peel ripeness (conductivity) b) The different ethanol yield of the banana peels at different ripeness (conductivity) c) Effect of filtration on Ethanol production (Filtration vs. Ethanol) 3. Are you comparing with a control or is a control built into your comparison? a) A control will be used to compare a filter to no filter, by running water through banana peel filters to test for heavy metals originating in the banana peel. b) A second control will be built into our experiment comparing the amount of ethanol produced with a filter, to the amount of ethanol produced without a filter. 4. How are you going to organize the data? Data will be organized into bins of relative ripeness. Within these ranges a certain proportion of the bananas will have their filtration ability as well as their ethanol yield measured and recorded (a data point for each respective banana). The remaining portion of bananas will have only their ethanol yield measured and recorded. This data will be organized into a table. 5. How many data points do you need to do an analysis? There is no minimum number of data points. 6. What statistical test will you use and why? N/A