Chemistry 11 Final Exam Review Sheet
advertisement
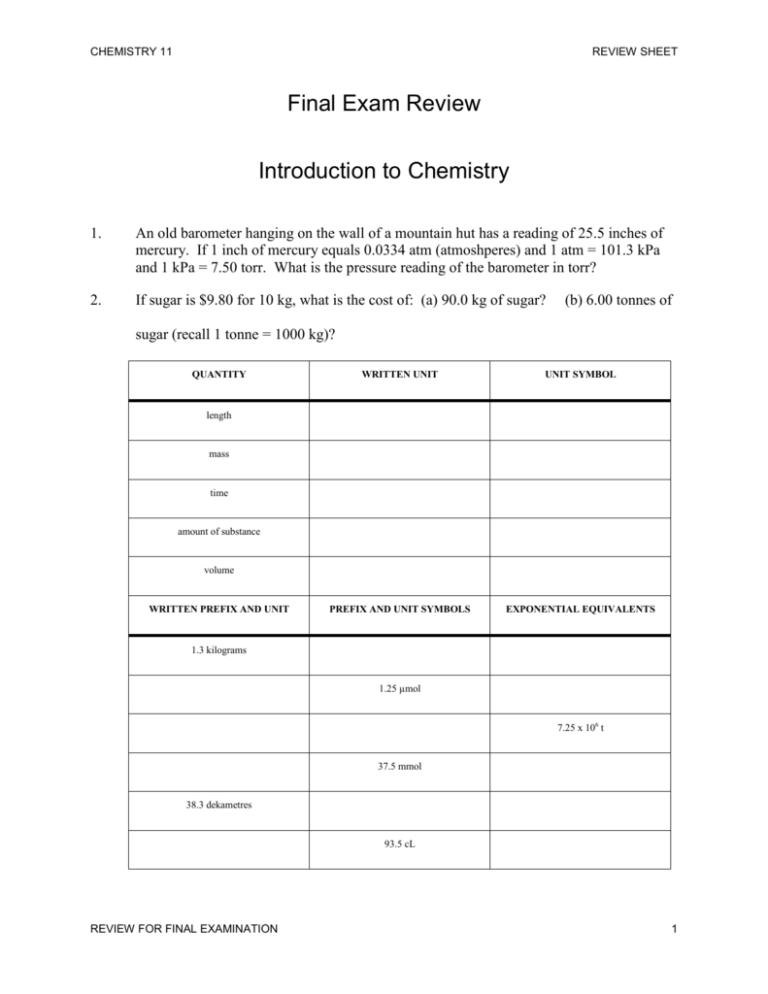
CHEMISTRY 11 REVIEW SHEET Final Exam Review Introduction to Chemistry 1. An old barometer hanging on the wall of a mountain hut has a reading of 25.5 inches of mercury. If 1 inch of mercury equals 0.0334 atm (atmoshperes) and 1 atm = 101.3 kPa and 1 kPa = 7.50 torr. What is the pressure reading of the barometer in torr? 2. If sugar is $9.80 for 10 kg, what is the cost of: (a) 90.0 kg of sugar? (b) 6.00 tonnes of sugar (recall 1 tonne = 1000 kg)? QUANTITY WRITTEN UNIT UNIT SYMBOL PREFIX AND UNIT SYMBOLS EXPONENTIAL EQUIVALENTS length mass time amount of substance volume WRITTEN PREFIX AND UNIT 1.3 kilograms 1.25 µmol 7.25 x 106 t 37.5 mmol 38.3 dekametres 93.5 cL REVIEW FOR FINAL EXAMINATION 1 CHEMISTRY 11 3. Convert the following: (a) 4. REVIEW SHEET 2.25 mL into L (c) 3125 ML into kL (e) 25 cm/µs into km/s If 1 L of granite has a mass of 5.50 kg, (a) what is the mass of 7.00 L of granite? (b) what is the volume occupied by 22 kg of granite? 5. A student measured the volume of an iron nail to be 0.880 mL and found that the mass was 6.92 g. What is the density of the iron? 6. A sample of vegetable oil had a density of 0.916 g/mL. Calculate the mass of 0.250 L of the oil. 7. What volume would 2.86 g of silver occupy? The density of silver is 10.5 g/mL. 8. Write the following numbers in scientific notation: (a) 9. (c) 21 700 000 (e) 95 007 000 (e) 2.57 x 106 Write the following numbers in decimal notation: (a) 10. 23 000 2.25 x 103 (c) 3.125 x 10-4 How many significant figures does each of the following measurements have? (a) 3218.04 cm (c) 6.84 x 10-4 mmol (e) 2500 s (b) 250.000 mL (d) 9 000 000 µs (f) 5.2500 x 10-6 cg REVIEW FOR FINAL EXAMINATION 2 CHEMISTRY 11 11. Determine the volumes of the following graduated cylinders: 12. Determine the reading on the following scales: REVIEW SHEET 13 State the rule for rounding to the correct number of significant figures after multiplying or dividing numbers. REVIEW FOR FINAL EXAMINATION 3 CHEMISTRY 11 14. REVIEW SHEET Perform the indicated operations and the give the answer to the correct number of significant figures. 15. (a) 35.8 x 0.12 (d) 1750 x (6.7254 x 102) (b) 128.62 ÷ 9.25 (e) (6.1428 x 103) ÷ 0.004810 In the following mixed calculations perform multiplications and divisions before doing the additions and subtractions. Keep track of the number of significant figures at each stage of a calculation. (a) 65.00 x 0.24000 – 15.78 x 0.148 (d) 16. Which of the following statements describe physical properties and which describe chemical properties? (a) glass is transparent (d) copper conducts electricity (b) salt melts at 801°C (e) fumes from ammonia and (c) Adding lye to fat makes soap hydrochloric acid mix to produce a white smoke 17. Which of the following are intensive properties and which are extensive? (a) shape (c) length (e) time to dissolve (b) smell (d) colour (f) density 18. Briefly describe the characteristics of solids, liquids, and gases. 19. Classify each of the following as one of element, compound, solution, or mechanical mixture. (a) gravel (d) iron (g) orange juice (b) coffee (e) water (h) ammonia REVIEW FOR FINAL EXAMINATION 4 CHEMISTRY 11 20. 21. REVIEW SHEET Classify each of the following as one of an atom, molecule, or ion. (a) NH3 (c) Pb (e) SO42- (b) Cr2O72- (d) PCl5 (f) Co How can you separate all the components in a mixture containing sand, iron filings, water, gasoline, red water-soluble dye, and blue water-soluble dye? In pure form the dyes are powders. 22. Which of the following represents the cooling curve for a pure substance. Explain how you know. 23. Classify each of the following as either a chemical (primarily) or physical change. (a) formation of fog (d) rusting nail (b) burning paper (e) dissolving salt into water (c) plant growing (f) filtering sand and water 24. List 4 characteristics of metals. 25. List 4 characteristics of nonmetals. REVIEW FOR FINAL EXAMINATION 5 CHEMISTRY 11 26. 27. Indicate which terms apply to each species. There is more than one term which applies to each species. N (neutral) C (cation) A (anion) M (monatomic) D (diatomic) P (polyatomic) (a) SO42- (c) Ba2+ (e) N2H5+ (b) NH3 (d) ClO- (f) Fe Write the formulae for the following ionic compounds. (a) 28. (b) Na2HPO4 diphosphorus trichloride (b) oxygen diiodide S4N2 (b) ClF3 zinc perchlorate hexahydrate (b) iron (III) sulphate nonhydrate (c) Co3(PO4)2•8H2O Name the following hydrated compounds. (a) 33. Ag3PO4 Write the formulae for the following hydrated compounds. (a) 32. uranium (IV) sulphate Name the following covalent compounds. (a) 31. (b) Write the formulae for the following covalent compounds. (a) 30. calcium dihydrogen phosphate Name the following ionic compounds. (a) 29. REVIEW SHEET FeSO4•5H2O Write the formulae for the following acids. (a) sulphuric acid (c) acetic acid (b) nitric acid (d) hydrochloric acid REVIEW FOR FINAL EXAMINATION 6 CHEMISTRY 11 34. REVIEW SHEET Name the following acids. (a) HF (c) H3PO4 (b) H2SO3 (d) HNO2 The Mole Concept 35. Review the following terms: arbitrary mass, Avogadro’s hypothesis, mole, atomic mass, molar mass, molar volume, STP, density, empirical formula, molecular formula, empirical mass, concentration, dilution, molarity. 36. Calculate the molar mass of each of the following. NCl3 (a) 37. NiSO4•7H2O (b) Cr(NO3)3•9H2O (b) 5.64 x 10-5 mol of AuCl3 (b) 6.48 kg of KMnO4 Calculate the mass of the following. (a) 39. Al2(SO4)3 Calculate the molar mass of each of the following. (a) 38. (b) 4.50 mol of PCl3 Calculate the number of moles in the following. (a) 85.6 g of CaO 40. Calculate the molar mass of 0.00496 mol sample of cholesterol has a mass of 1.894 g 41. What is STP and what are the experimental conditions of STP? REVIEW FOR FINAL EXAMINATION 7 CHEMISTRY 11 42. Calculate the volume at STP occupied by the following. (a) 43. 645 mL of SO2(g) 1 molecule of CH3CO2H (b) 2.56 mol of (NH4)3PO4 1 Pb atom (b) 5.62 x 1018 Fe(OH)3 molecules 60.5 g of AlCl3 (b) 84.6 mL of HCl(g) at STP 8.27 x 1020 molecules of O2(g) (b) 125.0 g of Cl2(g) Calculate the percentage composition of the following. (a) 49. (b) What volume at STP is occupied by each of the following? (a) 48. 64.8 L of Xe(g) How many atoms are contained in each of the following? (a) 47. 0.0861 mol of HCl Find the mass, in grams, of each of the following. (a) 46. (b) How many atoms are contained in the following. (a) 45. 24.8 mol of NH3 Calculate the number of moles in the following gases at STP. (a) 44. REVIEW SHEET NaHCO3 (b) CuSO4•5H2O Calculate the percentage composition of the bold species in each of the following. (a) Cr(NO3)6Cl3•H2O REVIEW FOR FINAL EXAMINATION (b) Al2(SO4)3•18H2O 8 CHEMISTRY 11 50. Find the empirical formula for the following compounds. (a) 51. REVIEW SHEET 12.6% Li, 29.2% S, 58.2% O (b) 38.8% Fe, 16.7% C, 44.5% O A gas has the empirical formula CH2. If 0.550 L of the gas at STP has a mass of 3.44 g, what is the molecular formula? 52. A sample of gas is analyzed and found to contain 33.0% Si and 67.0% F. If the gas has a density of 7.60 g/L at STP, what is the molecular formula? 53. Calculate the molar concentration of the following solutions. 54. 55. (a) 0.578 mol of NaCl in 52.0 mL of solution (b) 50.0 g of Fe(NO3)3 in 150.0 mL of solution Calculate the mass of solute needed to make the following solutions. (a) 125.0 mL of 0.0750 M KOH, from solid KOH (b) 500.0 mL of 0.120 M FeCl3, from solid FeCl3•6H2O What is the concentration of the solution that results when 250.0 mL of water is added to 550.0 mL of 3.50 M NaOH? 56. If 500.0 mL of 0.100 M LiOH is boiled down to 200.0 mL, what is the concentration? 57. What is the resulting concentration when 500.0 mL of 0.250 M NaCl is mixed with 250.0 mL of 0.450 M NaCl and the mixture is boiled down to 400.0 mL? 58. If 250.0 mL of solution A containing 28.0 g of LiOH is mixed with 500.0 mL of solution B containing 56.0 g of LiOH and the resulting solution is boiled down to 600.0 mL, what is the concentration? Unit VI Chemical Reactions 59. How can you tell that a chemical reaction has occurred? REVIEW FOR FINAL EXAMINATION 9 CHEMISTRY 11 REVIEW SHEET 60. What is the Law of Conservation of Mass? 61. How can you tell that a chemical equation is balanced? 3. Balance the following chemical reactions: 62. A. ___ Si4H10 + ___ O2 ___ SiO2 + ___ H2O B. ___ Ca3(PO4)2 + ___ SiO2 + ___ C ___ CaSiO3 + ___ CO + ___ P4 C. ___ C3H7N2O7 + ___ O2 ___ CO2 + ___ H2O + ___ N2 Write and balance the following word equations: A. Aluminum + Copper (II) sulphate Aluminum sulphate + Copper __________________________________________________________________ B. Magnesium nitride + Water Magnesium hydroxide + Ammonia (NH3) __________________________________________________________________ C. Calcium hydroxide + Ammonium chloride Ammonia + Calcium chloride + Water __________________________________________________________________ 63. Complete and balance the following reactions and classify each equation as one of: synthesis, decomposition, single replacement, double replacement, neutralization or combustion. A. ___ HF + ___ Fe(OH)3 ____________________________________________ B. ___ FeCl2 + ___ K2S ______________________________________________ C. ___ Al + ___ S8 __________________________________________________ D. ___ N2O _______________________________________________________ E. ___ C3H6OS2 + ___ O2 ____________________________________________ F. ___ Mg + ___ HCl _______________________________________________ REVIEW FOR FINAL EXAMINATION 10 CHEMISTRY 11 64. 65. REVIEW SHEET Define the terms: A. Exothermic B. Endothermic Classify the following is exothermic or endothermic: A. 2Na + 2H2O 2NaOH + H2 + 283 kJ B. KClO3 + 41.4 kJ K+ + ClO3– C. C2H6 + O2 CO2 + H2O D. 12CO2 + 11H2O C12H22O11 + 12O2 ∆H = – 3718 kJ ∆H = +5638 kJ Unit VII Stoichiometry 66. What are limiting and excess reactants? 67. Consider the reaction: 4C4H9SO2 + 25O2 16CO2 + 18H2O + 4SO2 68. A. How many oxygen molecules react with 20 molecules of C4H9SO2? B. How many moles of C4H9SO2 are required to produce 100 moles of water? C. What mass of SO2 is formed when 50.0 g of C4H9SO2 is reacted? A 25.0 mL sample of Al(OH)3 is titrated with 67.8 mL of 0.450 M HCl according to the reaction Al(OH)3 + 3HCl AlCl3 + 3H2O What is the concentration of the original Al(OH)3 solution? REVIEW FOR FINAL EXAMINATION 11 CHEMISTRY 11 69. REVIEW SHEET What mass of CS2 is produced when 25.8 g of C are reacted with 54.2 g of SO2 according to the equation 5C + 2SO2 CS2 + 4CO 70. A. What mass of CS2 is produced? B. What mass of the excess reactant will be left over? Consider the reaction K2Cr2O7 + 6NaI + 7H2SO4 Cr2(SO4)3 + 3I2 + 7H2O + 3Na2SO4 + K2SO4 A 35.0 g sample of pure K2Cr2O7 produces 9.67 g of H2O. What is the percentage yield? Unit VIII Atoms, Periodic Table, and Bonding 71. Which model of the atom is described by the following: A. Dense positively charged nucleus with electrons orbiting like planets around the sun. B. Correctly predicts the spectrum of hydrogen. C. Plum pudding model. REVIEW FOR FINAL EXAMINATION 12 CHEMISTRY 11 D. 72. REVIEW SHEET Atoms are like small spherical balls of matter. Fill in the following table: SYMBOL PROTONS NEUTRON ELECTRONS Rh 107 Pd2+ 123 73. 74. 75. Sb3– 93 89 50 46 Write the electron configurations for the following: (a) Mo (b) Bi (c) Bk (d) Ag+ (e) As3– (f) Pb4+ State the chemical family or group to which each of the following elements belongs. (a) silver (d) xenon (g) silicon (b) calcium (e) potassium (h) promethium (c) iodine (f) californium (i) tungsten Consider the two atoms Sn and I. (a) Which atom has the larger atomic radius? (b) Which atom has the larger ionization energy? (c) Which atom has more shells? (d) How many valence electrons each atom have? (e) What is the valence of each atom? REVIEW FOR FINAL EXAMINATION 13 CHEMISTRY 11 (f) 76. Na and O (b) H and C (c) N and F Which member of each following pair would you expect to have the higher melting point? (a) 78. Which atom has a greater electrostatic attraction between its nucleus and outermost electrons? Which of the following pairs would you expect to form ionic bonds and which would you expect to form covalent bonds? (a) 77. REVIEW SHEET MgO and CsCl (b) LiF and BeO (c) MgS and BaS (c) PF5 Predict the shape of the following molecules. (a) NCl3 (b) H2O Unit X Organic Chemistry 79. Name the following compounds: A. C—C—C—C—C—C—C—C | C—C—C—C B. C—C | C—C C—C—C—C—C—C | | C—C—C C—C __________________________________ __________________________________ C. D. Br F C—C—C | | | C—C—C—C—C—C—C—C | | | Br F C—C F F | | C—C C—C—C—C—C—C | F __________________________________ __________________________________ E. F. C—C—C—C | Br \ C—C—C—C—C—C / —C—C—C—C \ C—C—C __________________________________ REVIEW FOR FINAL EXAMINATION | Br __________________________________ 14 CHEMISTRY 11 80. 81. 82. REVIEW SHEET Draw the following compounds (draw carbon skeletons only). A. 2,2,4,4–tetramethylheptane B. 3,4–diethyl–3–methyl–1–heptyne Draw the following compounds (draw carbon skeletons only). A. 1,3–dipropylcyclopentane B. 1,1,1–trifluoro–2–methylpentane Draw the following compounds A. 3–methyl–1–hexanol REVIEW FOR FINAL EXAMINATION B. 1, 2,4–trimethyl benzene 15 CHEMISTRY 11 83. REVIEW SHEET Name the following geometric isomers (remember to include cis or trans as appropriate) A. C—C—C C—C \ / C=C / \ C C—C—C __________________________________ 84. B. C—C—C \ C=C C—C / \ | C C—C—C—C __________________________________ (A) Name the functional groups: Carboxyllic acid Ester Aromatic ring Halide Alcohol Aldehyde Ketone Ether Amine Amide A. C–C–C–C–C–C–O–C–C B. O || / C \ C–C–C ________________________________ ________________________________ C. D. F–C–C = C–C–C–C–F | C F C | | C–C–C–C–C–C–CO–C–C | | C C ________________________________ ________________________________ E. F. –CHO C C O | | || C–C–C–C–C–C–C–C–C | | C C–C–C ________________________________ ________________________________ G. H. C–C–C–C–C–COO–C REVIEW FOR FINAL EXAMINATION C–C–C–C–C–C–C–NH2 | | NH2 NH2 16 CHEMISTRY 11 REVIEW SHEET ________________________________ ________________________________ I. J F | C–C–C–C–C–C–C–COOH | | F C ________________________________ REVIEW FOR FINAL EXAMINATION OH | | C | C ________________________________ 17 CHEMISTRY 11 ANSWERS TO REVIEW SHEET Answers 1. An old barometer hanging on the wall of a mountain hut has a reading of 25.5 inches of mercury. If 1 inch of mercury equals 0.0334 atm (atmoshperes) and 1 atm = 101.3 kPa and 1 kPa = 7.50 torr. What is the pressure reading of the barometer in torr? 647 torr 2. If sugar is $9.80 for 10 kg, what is the cost of: (a) 90.0 kg of sugar? $88.20 (b) 6.00 tonnes of sugar (recall 1 tonne = 1000 kg)? $5.88E3 QUANTITY WRITTEN UNIT UNIT SYMBOL length Metre M mass g g time Second S amount of substance Mole Mol volume Litre L WRITTEN PREFIX AND UNIT PREFIX AND UNIT SYMBOLS EXPONENTIAL EQUIVALENTS 1.3 kilograms 1.3 kg 103 1.25 micromoles 1.25 µmol 1.25 E -6 7.25 megatonnes 7.25 Mt 7.25 x 106 t 37.5 millimoles 37.5 mmol 37.5E-3 mol 38.3 dekametres 38.3dam 38.3 E1 m 93.5 centilitres 93.5 cL 93.5 E-2 L REVIEW FOR FINAL EXAMINATION 1 CHEMISTRY 11 3. ANSWERS TO REVIEW SHEET Convert the following: (a) 2.25 mL into L (c) 2.25E-3L 4. 3125 ML into kL (e) 25 cm/µs into km/s 3.125E6 kL 2.5 E 2 km/s If 1 L of granite has a mass of 5.50 kg, (a) what is the mass of 7.00 L of granite? 38.5 kg (b) what is the volume occupied by 22 kg of granite? 4L 5. A student measured the volume of an iron nail to be 0.880 mL and found that the mass was 6.92 g. What is the density of the iron? 7.86 g/mL 6. A sample of vegetable oil had a density of 0.916 g/mL. Calculate the mass of 0.250 L of the oil. 229g 7. What volume would 2.86 g of silver occupy? The density of silver is 10.5 g/mL.. 0.272mL 8. Write the following numbers in scientific notation: (a) 23 000, 2.3E4 (c) 21 700 000, 2.17E7 (e) 95 007 000, 9.5007E7 9. Write the following numbers in decimal notation: (a) 2.25 x 103, 2250 (c) 3.125 x 10-4, 0.0003125 10. 2.57 x 106 (e) 2 570 000 How many significant figures does each of the following measurements have? (a) 3218.04 cm 6 (c) 6.84 x 10-4 mmol 3 (e) 2500 s 2 (b) 250.000 mL 6 (d) 9 000 000 µs 1 (f) 5.2500 x 10-6 cg 5 REVIEW FOR FINAL EXAMINATION 2 CHEMISTRY 11 11. ANSWERS TO REVIEW SHEET Determine the volumes of the following graduated cylinders: 54.2+/-.1 12. 82+/-1 38.0+/-.5 Determine the reading on the following scales: 15.17+-.01 10.0+-.02 6.40+-.05 15.69+-.01 16.4+-.02 7.60+-.05 13 State the rule for rounding to the correct number of significant figures after multiplying or dividing numbers. Lowest number for mult and div, only one uncertain column for add and sub REVIEW FOR FINAL EXAMINATION 3 CHEMISTRY 11 14. ANSWERS TO REVIEW SHEET Perform the indicated operations and the give the answer to the correct number of significant figures. (a) 35.8 x 0.12 =4.3 (d) 1750 x (6.7254 x 102)=1.18E6 (b) 128.62 ÷ 9.25=13.9 (e) (6.1428 x 103) ÷ 0.004810= 1.277E6 15. In the following mixed calculations perform multiplications and divisions before doing the additions and subtractions. Keep track of the number of significant figures at each stage of a calculation. (a) 65.00 x 0.24000 – 15.78 x 0.148 (d) =3E2 =13.26 16. Which of the following statements describe physical properties and which describe chemical properties? (a) glass is transparent P (d) copper conducts electricity P (b) salt melts at 801°C P (e) fumes from ammonia and (c) Adding lye to fat makes soap C hydrochloric acid mix to produce a white smoke C 17. 18. Which of the following are intensive properties and which are extensive? (a) shape e (c) length e (e)time to dissolve e (b) smell i (d) colour i (f) density i Briefly describe the characteristics of solids (fixed shape and volume, particles touching with only vibrations), liquids (fixed volume, particles can slide past one another, and gases (particles move quickly and are separated by lots of space). REVIEW FOR FINAL EXAMINATION 4 CHEMISTRY 11 19. ANSWERS TO REVIEW SHEET Classify each of the following as one of element, compound, solution, or mechanical mixture. 20. 21. (a) gravel m (d) iron e (g) orange juice m (b) coffee s (e) water c (h) ammonia c Classify each of the following as one of an atom, molecule, or ion. (a) NH3 m (c) Pb a (e) SO42- i (b) Cr2O72- i (d) PCl5 m (f) Co a How can you separate all the components in a mixture containing sand, iron filings, water, gasoline, red water-soluble dye, and blue water-soluble dye? In pure form the dyes are powders…magnet (iron), filter (sand), sep funnel (gas), evaporate (water), chromatography (dyes) REVIEW FOR FINAL EXAMINATION 5 CHEMISTRY 11 22. ANSWERS TO REVIEW SHEET Which of the following represents the cooling curve for a pure substance. Explain how you know. c cooling and plateus at phase change 23. Classify each of the following as either a chemical (primarily) or physical change. (a) formation of fog p (d) rusting nail c (b) burning paper c (e) dissolving salt into water p (c) plant growing c (f) filtering sand and water p 24. List 4 characteristics of metals. Shiny, ductile, malleable, conductor 25. List 4 characteristics of nonmetals. Brittle, dull, poor conductor, usually gas 26. Indicate which terms apply to each species. There is more than one term which applies to each species. N (neutral) C (cation) A (anion) M (monatomic) D (diatomic) P (polyatomic) (a) A, P, SO42- (c) C, M, Ba2+ (e) C, P, N2H5+ (b) N, P, NH3 (d) A, D, ClO- (f) N, M, Fe REVIEW FOR FINAL EXAMINATION 6 CHEMISTRY 11 27. Write the formulae for the following ionic compounds. (a) 28. ANSWERS TO REVIEW SHEET calcium dihydrogen phosphate (b) Ca2(H2PO4)2 U(SO4)2 Name the following ionic compounds. (a) Ag3PO4 Silver phosphate 29. diphosphorus trichloride P2Cl3 S4N2 tetrasulphur dinitride zinc perchlorate hexahydrate Zn(ClO4)2.6H2O FeSO4•5H2O Iron (II) sulphate pentahydrate 34. (b) oxygen diiodide OI2 (b) ClF3 Chlorine trifluoride (b) iron (III) sulphate nonhydrate Fe2(SO4)3.9H2O Name the following hydrated compounds. (a) 33. sodium monohydrogen phosphate Write the formulae for the following hydrated compounds. (a) 32. Na2HPO4 Name the following covalent compounds. (a) 31. (b) Write the formulae for the following covalent compounds. (a) 30. uranium (IV) sulphate (b) Co3(PO4)2•8H2O Cobalt (II) phosphate octahydrate Write the formulae for the following acids. (a) sulphuric acid H2SO4 (c) acetic acid CH3COOH (b) nitric acid HNO3 (d) hydrochloric acid HCl Name the following acids. (a) HF hydrofluoric acid (c) H3PO4 phosphoric acid (b) H2SO3 sulphurous acid (d) HNO2 nitrous acid REVIEW FOR FINAL EXAMINATION 7 CHEMISTRY 11 ANSWERS TO REVIEW SHEET The Mole Concept 36. Calculate the molar mass of each of the following. NCl3 (b) 120.5g/mol 37. 342.3g/mol Calculate the molar mass of each of the following. (a) NiSO4•7H2O 280.8g/mol 38. 4.50 mol of PCl3 619 g Cr(NO3)3•9H2O 400.0g/mol (b) 5.64 x 10-5 mol of AuCl3 0.0171 g Calculate the number of moles in the following. (a) 85.6 g of CaO 1.53 mol 40. (b) Calculate the mass of the following. (a) 39. Al2(SO4)3 (c) (b) 6.48 kg of KMnO4 41.0 mol Calculate the molar mass of 0.00496 mol sample of cholesterol has a mass of 1.894 g. 382 g/mol 41. What is STP and what are the experimental conditions of STP? Standard temp (0-oC) and pressure (101.3 kPa) 42. Calculate the volume at STP occupied by the following. (c) 24.8 mol of NH3 556 L 43. 0.0861 mol of HCl 1.93L Calculate the number of moles in the following gases at STP. (a) 44. (c) 64.8 L of Xe(g)= 2.89 mol (b) 645 mL of SO2(g) = 2.88E-2 mol How many atoms are contained in the following. REVIEW FOR FINAL EXAMINATION 8 CHEMISTRY 11 (a) 1 molecule of CH3CO2H 8 45. (b) 2.56 mol of (NH4)3PO4 3.08E25 Find the mass, in grams, of each of the following. (a) 1 Pb atom 3.44E-22 g 46. ANSWERS TO REVIEW SHEET (b) 5.62 x 1018 Fe(OH)3 molecules 9.97E-4 g How many atoms are contained in each of the following? (a) 60.5 g of AlCl3= 1.09E24 (b) 84.6 mL of HCl(g) at STP= 4.55E21 47. What volume at STP is occupied by each of the following? (a) 8.27 x 1020 molecules of O2(g) 30.1mL 48. (b) 125.0 g of Cl2(g) 39.4L Calculate the percentage composition of the following. (a) Na (27.4)H (1.2)C (14.3) O3(57.1) (c) CuSO4•5H2O Cu-25.4, S-12.1, O-57.7, H-4.0 49. Calculate the percentage composition of the bold species in each of the following. (a) Cr(NO3)6Cl3•H2O 67.8% 50. Al2(SO4)3•18H2O 43.3% Find the empirical formula for the following compounds. (a) 12.6% Li, 29.2% S, 58.2% O Li2SO4 51. (b) (b) 38.8% Fe, 16.7% C, 44.5% O FeC2O4 A gas has the empirical formula CH2. If 0.550 L of the gas at STP has a mass of 3.44 g, what is the molecular formula? C10H20 REVIEW FOR FINAL EXAMINATION 9 CHEMISTRY 11 ANSWERS TO REVIEW SHEET 52. A sample of gas is analyzed and found to contain 33.0% Si and 67.0% F. If the gas has a density of 7.60 g/L at STP, what is the molecular formula? Si2F6 53. Calculate the molar concentration of the following solutions. 54. 55. (a) 0.578 mol of NaCl in 52.0 mL of solution. 11.1M (b) 50.0 g of Fe(NO3)3 in 150.0 mL of solution 1.38M Calculate the mass of solute needed to make the following solutions. (a) 125.0 mL of 0.0750 M KOH, from solid KOH 0.526g (b) 500.0 mL of 0.120 M FeCl3, from solid FeCl3•6H2O What is the concentration of the solution that results when 250.0 mL of water is added to 550.0 mL of 3.50 M NaOH? 2.41M 56. If 500.0 mL of 0.100 M LiOH is boiled down to 200.0 mL, what is the concentration? 0.25M 57. What is the resulting concentration when 500.0 mL of 0.250 M NaCl is mixed with 250.0 mL of 0.450 M NaCl and the mixture is boiled down to 400.0 mL?0.594M 58. If 250.0 mL of solution A containing 28.0 g of LiOH is mixed with 500.0 mL of solution B containing 56.0 g of LiOH and the resulting solution is boiled down to 600.0 mL, what is the concentration? 5.86M Unit VI Chemical Reactions 59. How can you tell that a chemical reaction has occurred? New properties due to new substances. (d) What is the Law of Conservation of Mass? Mass of products = mass of reactants in a closed reaction 60. How can you tell that a chemical equation is balanced? a. Equal #s of atoms on each side REVIEW FOR FINAL EXAMINATION 10 CHEMISTRY 11 61. 62. ANSWERS TO REVIEW SHEET Balance the following chemical reactions: A. 2 Si4H10 + 13 O2 8 SiO2 + 10 H2O B. 2 Ca3(PO4)2 + 6 SiO2 + 10 C 6 CaSiO3 + 10 CO + 1 P4 C. 4C3H7N2O7 + 5 O2 12 CO2 + 14 H2O + 4 N2 Write and balance the following word equations: A. Aluminum + Copper (II) sulphate Aluminum sulphate + Copper 2Al + 3CuSO4 1Al2(SO4)3 + 3Cu B. Magnesium nitride + Water Magnesium hydroxide + Ammonia (NH3) 1Mg3N2 + 6H2O 3Mg(OH)2 + 2NH3 C. Calcium hydroxide + Ammonium chloride Ammonia + Calcium chloride + Water 1Ca(OH)2 + 2NH4Cl 2NH3 + 1CaCl2 + 2H2O 63. 64. Complete and balance the following reactions and classify each equation as one of: synthesis, decomposition, single replacement, double replacement, neutralization or combustion. A. 3 HF + 1 Fe(OH)3 neut 3H2O + 1FeF3 B. 1 FeCl2 + 1K2S dr 1FeS + 2KCl C. 16 Al + 3 S8 syn 8Al2S3 D. 2N2O decom 2N2 + 1O2 E. 1C3H6OS2 + ___ O2 combust 3CO2 + 3H2O + 2SO2 F. 1 Mg + 2 HCl s.r. 1MgCl2 + 2H2 Define the terms: A. Exothermic Heat exits the system B. Endothermic Heat enters the system REVIEW FOR FINAL EXAMINATION 11 CHEMISTRY 11 65. ANSWERS TO REVIEW SHEET Classify the following is exothermic or endothermic: A. 2Na + 2H2O 2NaOH + H2 + 283 kJ ex B. KClO3 + 41.4 kJ K+ + ClO3– en C. C2H6 + O2 CO2 + H2O D. 12CO2 + 11H2O C12H22O11 + 12O2 ∆H = – 3718 kJ ex ∆H = +5638 kJ en Unit VII Stoichiometry 65. What are limiting (runs out and determines the amount of product) and excess (there is more than is needed to fully react with the limiting reactant )reactants? 66. Consider the reaction: 4C4H9SO2 + 25O2 16CO2 + 18H2O + 4SO2 A. How many oxygen molecules react with 20 molecules of C4H9SO2? 125 B. How many moles of C4H9SO2 are required to produce 100 moles of water? 22.2 C. 67. What mass of SO2 is formed when 50.0 g of C4H9SO2 is reacted? 26.5g A 25.0 mL sample of Al(OH)3 is titrated with 67.8 mL of 0.450 M HCl according to the reaction Al(OH)3 + 3HCl AlCl3 + 3H2O What is the concentration of the original Al(OH)3 solution? 0.407M 68. What mass of CS2 is produced when 25.8 g of C are reacted with 54.2 g of SO2 according to the equation 5C + 2SO2 CS2 + 4CO A. What mass of CS2 is produced? 32.2g B. What mass of the excess reactant will be left over? 0.4g C REVIEW FOR FINAL EXAMINATION 12 CHEMISTRY 11 69. ANSWERS TO REVIEW SHEET Consider the reaction K2Cr2O7 + 6NaI + 7H2SO4 Cr2(SO4)3 + 3I2 + 7H2O + 3Na2SO4 + K2SO4 A 35.0 g sample of pure K2Cr2O7 produces 9.67 g of H2O. What is the percentage yield? 64.5% Unit VIII Atoms, Periodic Table, and Bonding 70. 71. Which model of the atom is described by the following: A. Dense positively charged nucleus with electrons orbiting like planets around the sun. rutherford B. Correctly predicts the spectrum of hydrogen. bohr C. Plum pudding model. thompson D. Atoms are like small spherical balls of matter. Dalton Fill in the following table: SYMBOL PROTONS NEUTRON ELECTRONS Rh 45 58 45 46 61 44 Sb3– 51 72 54 Np4+ 93 144 89 50 69 46 107 Pd2+ 123 237 119 72. 73. Sn4+ Write the electron configurations for the following: (a) Mo= [Kr]5s24d4 (b) Bi =[Xe] 6s24f14 5d10 6p3 (c) Bk=[Rn]7s26d15f8 (d) Ag+ =[Kr] 5s1 4d9 or 4d10 like copper (e) As3–=[Ar]4s23d104p6 (f) Pb4+= 4f14 5d10 State the chemical family or group to which each of the following elements belongs. REVIEW FOR FINAL EXAMINATION 13 CHEMISTRY 11 74. 75. (a) silver trans (d) xenon noble (g) silicon rep (b) calcium alk earth (e) potassium alk met (h) promethium lanth (c) iodine halo (f) californium Act (i) tungsten trans Consider the two atoms Sn and I. (a) Which atom has the larger atomic radius? Sn (b) Which atom has the larger ionization energy? I (c) Which atom has more shells? tie (d) How many valence electrons each atom have? Sn 4, I 7 (e) What is the valence of each atom? Sn 4, I 1 (f) Which atom has a greater electrostatic attraction between its nucleus and outermost electrons? I Which of the following pairs would you expect to form ionic bonds and which would you expect to form covalent bonds? (a) 76. Na and O Ionic (b) H and C covalent (c) N and F covalent Which member of each following pair would you expect to have the higher melting point? (a) 77. ANSWERS TO REVIEW SHEET MgO and CsCl (b) LiF and BeO (c) MgS and BaS (c) PF5 trigonal bipyramid Predict the shape of the following molecules. (a) NCl3 trigonal pyramid (b) H2O bent Unit X Organic Chemistry REVIEW FOR FINAL EXAMINATION 14 CHEMISTRY 11 78. ANSWERS TO REVIEW SHEET Name the following compounds: A. C—C—C—C—C—C—C—C | C—C—C—C 4, 6-diethyl-4-propyl-2-octyne C. D. Br F C—C—C | | | C—C—C—C—C—C—C—C | | | Br F C—C C—C—C—C | E. \ C—C—C 1-butyl-3-propylcyclopentane F F | | C—C C—C—C—C—C—C | F 4,5,6-trifluoro-2-octyne F. Br \ C—C—C—C—C—C / —C—C—C—C | Br 1,5-dibromo-2-butyl-3-hexylbenzene Draw the following compounds (draw carbon skeletons only). A. 2,2,4,4–tetramethylheptane CH3 H3C B. CH3 CH3 CH3 80. C—C | C—C C—C—C—C—C—C | | C—C—C C—C 5-propylnonane 1,2-dibromo-6-ethyl-2,4-difluoro-4propyloctane 79. B. CH3 3,4–diethyl–3–methyl–1–heptyne Draw the following compounds (draw carbon skeletons only). REVIEW FOR FINAL EXAMINATION 15 CHEMISTRY 11 A. ANSWERS TO REVIEW SHEET 1,3–dipropylcyclopentane H3C CH3 B. 1,1,1–trifluoro–2–methylpentane F CH3 F F CH3 81. Draw the following compounds A. 3–methyl–1–hexanol CH3 CH3 H3C HO 82. CH3 1, 2,4–trimethyl benzene CH3 Name the following geometric isomers (remember to include cis or trans as appropriate) A. C—C—C C—C \ / C=C / \ C C—C—C 4-ethyl-5-methyl-trans-4-octene 83. B. B. C—C—C \ C=C C—C / \ | C C—C—C—C 7-ethyl-4-methyl-trans-4-nonene (A) Name the functional groups: REVIEW FOR FINAL EXAMINATION 16 CHEMISTRY 11 ANSWERS TO REVIEW SHEET Carboxyllic acid Ester Aromatic ring Halide Alcohol Aldehyde Ketone Ether Amine Amide A. C–C–C–C–C–C–O–C–C B. O || / C Ether \ C–C–C ketone C. F–C–C = C–C–C–C–F | C D. F C | | C–C–C–C–C–C–CO–C–C | | C C Halide Halide, ketone E. F. –CHO C C O | | || C–C–C–C–C–C–C–C–C | | C C–C–C Aldehyde and aromatic ring ketone G. H. C–C–C–C–C–COO–C ester I. C–C–C–C–C–C–C–NH2 | | NH2 NH2 amine F | C–C–C–C–C–C–C–COOH | | F C Carboxylic acid, halide REVIEW FOR FINAL EXAMINATION J OH | | C | C alcohol 17





