Words
advertisement

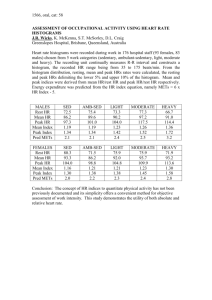
43 Journal of Exercise Physiologyonline October 2014 Volume 17 Number 5 Editor-in-Chief Official Research Tommy Journal Boone, of thePhD, American MBA Review Board Society of Exercise Todd Astorino, Physiologists PhD Julien Baker, PhD Steve Brock, ISSN 1097-9751 PhD Lance Dalleck, PhD Eric Goulet, PhD Robert Gotshall, PhD Alexander Hutchison, PhD M. Knight-Maloney, PhD Len Kravitz, PhD James Laskin, PhD Yit Aun Lim, PhD Lonnie Lowery, PhD Derek Marks, PhD Cristine Mermier, PhD Robert Robergs, PhD Chantal Vella, PhD Dale Wagner, PhD Frank Wyatt, PhD Ben Zhou, PhD Official Research Journal of the American Society of Exercise Physiologists ISSN 1097-9751 JEPonline The Relationship between Exercise-Induced and NonStimulated 24-Hr Growth Hormone Release Kevin Ritsche1, Jim Patrie2, Arthur Weltman3, Laurie Wideman4 1Department of Exercise Physiology, Winston-Salem State University, Winston-Salem, NC, 2Department of Health Evaluation Sciences, University of Virginia, Charlottesville, VA, 3General Clinical Research Center, University of Virginia, Charlottesville, VA, 4Department of Kinesiology, University of North Carolina at Greensboro, Greensboro, NC USA ABSTRACT Ritsche K, Patrie J, Weltman A, Wideman L. The Relationship between Exercise-Induced and Non-Stimulated 24-Hr Growth Hormone Release. JEPonline 2014;17(5):43-57. The purpose of this study was to quantify the relationship between the acute exerciseinduced growth hormone (GH) response, gender, fitness, age, and body composition with non-exercise stimulated (NES) 24-hr integrated GH concentration (IGHC). Twenty-nine subjects (16 males, 13 females) completed a 24-hr control session without exercise (NES) on one occasion and a 30-min exercise session on a separate occasion. The GH release was analyzed from 10-min intervals using trapezoidal integration during both sessions. Multiple regression analysis in males revealed that significant variability in NES 24-hr IGHC could be adequately explained by subject age and BMI together (P<0.05), but could not be explained by exercise-induced peak GH or 30-min of exercise-induced IGHC on an unrelated day (P=0.750). However, significant variability in NES 24-hr IGHC in females could be uniquely associated with subject age (P<0.05) as well as peak GH and 30-min of exercise-induced IGHC (P<0.05). The findings indicate that in the females only the constant load exercise-induced growth hormone response can adequately predict total 24-hr growth hormone output on a separate day without an exercise stimulus. The data further exemplify gender disparities in exercise-induced growth hormone release and the importance of exercise for females in regards to enhancing total 24-hr growth hormone concentrations. Key Words: Body Composition, Sex, Hormones, Obesity 44 INTRODUCTION Growth hormone (GH) has been linked to many health related benefits including maintenance of lean body mass as a result of increased lipolysis (2,11,30) and skeletal muscle adaptations (3,12). It is well known that exercise is a potent stimulator of GH release (6-8,13,18,19,25,28). In young males and females, it has been shown that exercise-induced GH release increases linearly with exercise intensity (13,14,27). Age, body composition, and fitness level are factors that influence GH release independently and concomitantly (1,4,7,9,10,15,17,21,26,28). The reported significant effect of fitness level on GH release appear to be gender-mediated and equally important as body composition (20,24,25) and, interestingly, several studies have reported the attenuation of GH in overweight and obese individuals regardless of gender (1,5,10). Gender-mediated differences in GH release exist throughout the entire submaximal range of exercise intensities, with more pronounced differences at higher intensities of exercise, although aerobic exercise has been shown to stimulate GH release equally at maximal intensities (28). Even though both genders display a similar pattern of exercise-induced GH secretion across a broad range of exercise intensities, females tend to release more GH at any given exercise intensity because of greater basal GH secretion (14,25,27,28). During increasing intensities of exercise, females tend to secrete greater GH masses per burst to accommodate for a decrease in the number of GH peaks observed (25,28) during increasing exercise durations beyond 60 min while males typically have a greater GH peak and GH mean value attributed to a greater GH pulse frequency (22). Females have also been shown to obtain a peak GH concentration sooner than males, which is likely due to the fact that they start at a higher basal GH level and reach similar GH maximum exercise values (14,25,27,28). Increasing exercise intensity and duration results in a greater slope in the GH response in males, which is likely to make up for lower basal GH concentrations (26). We have previously reported no differences in 24-hr IGHC between continuous or intermittent modes of exercise (25) and thus, we used the data from the 30-min continuous constant load exercise session only for this secondary analysis. To our knowledge, no studies have investigated the association of total non-stimulated 24-hr GH concentration with an independent and unrelated acute exercise-induced GH measurement on an unrelated day. Given the known potent role of exercise for stimulating GH release and the numerous beneficial effects of GH on metabolism and body composition, it would be prudent to better understand the relationship between the exercise-induced GH and 24-hr GH concentrations. Information obtained from this study may help clinicians, dieticians and exercise physiologists identify the importance of exercise in the enhancement of GH release and its subsequent metabolic benefits as well as any gender-mediated differences in confounding effects of such variables as gender, age, fitness level, and body composition. Therefore, the purpose of the current study was to investigate whether the exercise-induced GH response during a single bout of 30-min constant load exercise was related to total 24-hr GH concentration over an unrelated 24-hr period without exercise. In addition, the confounding effects of gender, age, fitness level, and body composition on the relationship between exercise-induced IGHC and total NES 24-hr IGHC during an unrelated day were examined. 45 METHODS Subjects Twenty-nine subjects (N = 16 males and N = 13 females) participated in this study. All subjects were considered sedentary, which was defined as less than two 30-min bouts of exercise per week. Each subject provided written informed consent approved by the University of Virginia Human Investigators Committee. Each subject was screened for contraindications to exercise and factors known to affect GH secretion, including hematological, renal, hepatic, metabolic, and thyroid function. The subjects were also nonsmokers and not on any systemic medications. All females were pre-menopausal, and none was currently taking contraceptives. The females were tested during the early follicular phase, sometime between day 3 and day 8 after the onset of menses. Descriptive characteristics are presented in Table 1. Procedures Maximal Exercise Testing Peak oxygen consumption (VO2 peak) and blood lactate threshold (LT) were determined from a continuously graded cycle ergometer test (SensorMedics, Yorba Linda, CA) using open circuit spirometry (SensorMedics, Model 2900z, Yorba Linda, CA). Each subject started with an initial workload of 60 watts (W) that was increased by 15 W every 3 min until volitional fatigue occurred. Blood lactate measurements were taken from a forearm vein at rest and during the last 15 sec of each stage (YSI 2700 select biochemistry analyzer; Yellow Springs Instruments, Yellow Springs, OH). VO2 peak was chosen as the highest attained VO2 value during the test. To determine LT, the blood lactate concentration was plotted against power output and the LT was then chosen as the highest power output obtained before the curvilinear increase in blood lactate concentration occurred. General Clinical Research Center Admissions All subjects refrained from exercise for a 24-hr period prior to being randomly admitted to the General Clinical Research Center (GCRC) on two separate, counterbalanced and randomized occasions. One 24-hr admission included a 30-min bout of constant load exercise and the other 24-hr admission that served as a non-exercise control (NES). Subjects consumed their evening meal shortly following admission to the GCRC at 5 p.m. Except for the exercise portion of the applicable admission, subjects were asked to remain sedentary. They were only allowed to read, work on computers, and watch television quietly in bed. They were only allowed to be mobile to use the bathroom. At 9 p.m. an intravenous catheter was inserted bilaterally into each forearm vein. At 11 p.m. the lights were turned out. The subjects fasted overnight so that normal nocturnal GH release would not be affected by caloric consumption. The following morning, the subjects either exercised for 30-min on a treadmill (9:00 to 9:30 a.m.) with the exercise intensity set at a constant relative power output midway between the pre-determined VO2 at LT and VO2 peak (~74% VO2 peak, Table 1) or remained sedentary during the other counterbalanced, randomized admission. During this control admission, the subjects were admitted at the same time frame to the GCRC but did not exercise and the total NES 24-hr GH AUC value was used for comparison to the 30-min snapshot of exercise-induced GH release from the separate, unrelated admission. Subjects were given standardized snack at 8 p.m. the evening before testing (500 kcal, 55% carbohydrate, 15% protein, and 30% fat) as well as standardized meals the following day using strategic intervals at 10 a.m., 2 p.m., and 6 p.m. All meals had identical macronutrient content (55% carbohydrate, 15% protein, and 30% fat). Also, the meals were based on measured basal metabolic rate plus an activity factor for each subject. Each subjects had to consume the entire meal and the 46 diet, which contained no caffeine or Nutrasweet®. Sodium was controlled at 3 g. All subjects were allowed to consume water ad libitum. The data from two GCRC admissions was used to examine the relationship between the acute exercise-induced GH response from one randomized admission and 24-hr IGHC on a separate nonrelated admission where the subject did not exercise. This analysis was done to examine if 24-hr IGHC can be predicted from minimal blood sampling for GH during a 30-min exercise test, along with a host of other common GH predictor variables. Growth Hormone Measurements All measurements were conducted as previously described (25). Briefly, blood samples were taken from an intravenous catheter inserted into a forearm vein every 10 min during exercise and 6 hrs into recovery throughout both admissions. The GH concentrations were measured in duplicate from a single subject by a modified ultrasensitive chemiluminescence assay (Nichols Institute Diagnostics, San Clemente, CA) that had a sensitivity of 0.005 μg·L-1. Recombinant (22 kDa) human GH was used as the standard (6). Cross-reactivity with 20 kDa recombinant human GH was 30%. Median intra- and interassay coefficients of variation were 5.2% and 6.3%, respectively. Statistical Analyses Demographic and subject characteristic data were summarized by the mean (M) and standard deviation (SD) of the measurement distribution. Male versus female comparisons of age, weight, body mass index (BMI), measure of overall physical fitness, and GH responses were examined via a two-sample Wilcoxon rank-sum test. IGHC The 24-hr IGHC was calculated using the trapezoidal integration rule (21). Trapezoidal integration was also used to calculate integrated GH concentration for both 6-hr admissions that included a 30min bout of exercise. Since exercise alone had such a profound effect on GH release, inclusion of recovery time (1 to 6 hrs) beyond the 30 min of exercise-induced GH during the initial 30-min exercise time frame did not provide additional statistical information about 24-hr ICHC (post-exercise recovery time of 6 hrs only encompassed an additional 5% of total 24-hr IGHC) and thus, only the total 30-min IGHC resulting from the actual exercise session was used in the predictive model of the univariate and multivariate regression analyses along with the exercise-induced peak GH concentration (defined as the highest value attained during the 30 min of exercise). Univariate and Multiple Regression Analyses Within male and female subgroups, Spearman rank correlation analyses were conducted to examine bivariate relationships between the values of the predictor variables. Additionally, the ordinary leastsquares (OLS) univariate regression analysis was conducted to examine univariate relationships between 24-hr IGHC and: subject age, BMI, 30-min exercise-induced IGHC, exercise-induced peak GH concentration, and VO2 peak. Then, an OLS multivariate regression analysis was conducted to examine covariate adjusted relationships between NES 24-hr IGHC and: subject age, BMI, 30-min exercise-induced IGHC, exercise-induced peak GH concentration, and VO2 peak. In order to attain constant residual variance, as required by the OLS regression model constant variance assumption, the 24-hr IGHC measurements were rescaled to the natural logarithmic scale. Additionally, the natural logarithmic transformation was used to reduce the skewed nature of the 30-min IGHC measurement distribution and to also reduce the skewed nature of the exercise-induced peak GH concentration measurement distribution. With regard to hypothesis testing for the univariate regression analysis, the null hypothesis that there was no predictor variable versus response variable association was tested via the conventional 1 numerator degree freedom F-test for the univariate regression analyses. With 47 regard to hypothesis testing for the multivariate analysis, the null hypothesis that there was no unique association between loge(24-hr IGHC) and the predictor variable was tested via a Type III extra sum of squares F-test, which took into account the variance in the log e(24-hr IGHC) measurements that was explained by the remaining set of predictor variables. A P≤0.05 decision rule was used as the null hypothesis rejection criterion. The coefficient of determination R2 was used to quantify the proportion of the total variation in the loge(24-hr IGHC) measurements that was explained by the values of the predictor variable for both regression models. Spotfire Splus version 8.2 (TIBCO, Inc, Palo Alto, CA) was used to conduct all the statistical analyses. RESULTS Males and females did not differ significantly in age, weight, body mass index (BMI), or VO2 peak (Table 1). Exercise intensity for the 30-min exercise session was significantly greater in the males versus the females (24.7 ± 1.7 vs. 18.7 ± 2.3 mL·kg-1·min-1, P = 0.040). However, the load was relatively adjusted so that each subject exercised at a constant workload equal to ~74% of VO2 peak. Table 1. Descriptive and Maximal Exercise Testing Data Between Genders. Variables Men Women Age (yrs) 27 ± 2 (18 – 39) 27 ± 2 (17 – 40) P-value 0.979 Weight (kg) 89.9 ± 4.3 (61.1 – 127.7) 78.4 ± 5.4 (54.5 – 123) 0.122 BMI (kg·m-2) 28.5 ± 1.7 (20.2 – 45.8) 29.1 ± 2.4 (20.1 – 46.3) 0.824 VO2@LT (mL·kg-1·min-1) 16.0 ± 1.0 (9.3 – 22.9) 12.6 ± 1.5 (6.4 – 26.3) 0.060 HR@LT (beats·min-1) 122 ± 6 (80 – 168) 126 ± 3 (110 – 150) 0.511 Power@LT (W) 77.2 ± 4.8 (35 – 110) 52.3 ± 8.8 (20 – 125) 0.015* VO2 Peak (mL·kg-1·min-1) 33.4 ± 2.5 (16.4 – 47.4) 26.3 ± 3.0 (13.9 – 46.9) 0.073 176 ± 6 (131 – 200) 0.690 193.4 ± 11.8 (110 – 275) 131.9 ± 12.8 (65 – 215) 0.002 24.7 ± 1.7 (13.3 – 34.1) 18.7 ± 2.3 (6.9 – 36.6) 0.040* HR@VO2 Peak (beats·min-1) Power@VO2 Peak (W) 30-Min VO2 (mL·kg-1·min-1) 179 ± 5 (131 – 213) Means ± SD (range); N = 16 males; N = 13 females. LT = lactate threshold. 30-min VO2 (mL·kg-1·min-1) was pre-determined exercise intensity quantitatively described as the median between VO2@LT (mL·kg-1·min-1) and VO2 peak (mL·kg-1·min-1) in order to keep the relative workloads between genders equal. *P<0.05. The NES 24-hr IGHC was similar between genders (P = 0.488) as well as the 30-min exerciseinduced IGHC (P = 0.131) and peak GH concentration (P = 0.089) (Table 2). 48 Table 2. Total Exercise-Induced GH Concentration and Total Non-Exercise (NES) 24-Hr GH Concentration by Gender. Variables Men Women P-value 30-Min IGHC (µg·L-1· min-1) 448 ± 158 (20 – 2275) 750 ± 172 (61 – 1905) 0.131 Peak GH (µg·L-1) 9.1 ± 3.2 (0.1 – 40.4) 15.0 ± 3.4 (1.2 – 42.4) 0.089 1891 ± 418 (150 – 4911) 2353 ± 455 (212 – 5276) 0.488 NES 24-Hr IGHC (µg·L-1·min-1 ) Means ± SD (range); N = 16 males and N = 13 females. 10 10 9 9 9 8 7 6 5 log(24-hr GH AUC) 10 log(24-hr GH AUC) log(24-hr GH AUC) When examining the NES 24-hr IGHC data in males, univariate analyses showed that it was related to BMI (r2 = 0.50, P = 0.002) and age (r2 = 0.31, P = 0.010), but only marginally with 30-min of exercise-induced IGHC (r2 = 0.24, P = 0.051), and unrelated to exercise-induced peak GH concentration (r2 = 0.17, P = 0.122) and VO2 peak on a separate occasion (r2 = 0.14, P = 0.152) (Figure 1). 8 7 6 5 R2 = 0.31 P = 0.010 a 4 15 20 25 30 35 4 40 log(24-hr GH AUC) log(24-hr GH AUC) 9 8 7 6 5 6 23 28 33 38 43 4 48 BMI 9 4 R2 = 0.50 P = 0.002 b 18 10 7 5 Age 10 8 R2 = 0.24 P = 0.051 c 2 3 4 5 6 7 8 9 log(30-min Exercise GH AUC) 8 7 6 5 R2 = 0.17 P = 0.122 d -3 -2 -1 0 1 2 3 4 log(Peak Exercise GH) 4 5 R2 = 0.14 P = 0.152 e 10 15 20 25 30 35 40 45 50 VO2 Peak Figure 1. Univariate Relationships between loge(24-Hr GH AUC) and: Subject Age, BMI, loge(30-Min Exercise GH AUC), loge(Peak Exercise GH) and VO2 Peak , Within the Male Subgroup. R2 denotes the proportion of the total variation in the loge(24-hr GH AUC) values that is explained by the predictor variable values, and P denotes the P-value for the test of the null hypothesis that there is no association between the log e(24-hr GH AUC) values and the predictor variable values. 49 10 10 9 9 9 8 7 6 5 8 7 6 5 R2 = 0.31 P = 0.049 4 15 20 25 30 35 40 18 23 28 33 38 43 48 BMI 10 9 9 log(24-hr GH AUC) 10 8 7 6 5 8 7 6 5 R2 = 0.60 P = 0.002 4 Age log(24-hr GH AUC) log(24-hr GH AUC) 10 log(24-hr GH AUC) log(24-hr GH AUC) In females, significant univariate relationships were found between NES 24-hr IGHC and age (r2 = 0.31, P = 0.049), BMI (r2 = 0.60, P = 0.002), 30-min of exercise-induced IGHC (r2 = 0.83, P < 0.001), exercise-induced peak GH concentration (r2 = 0.63, P = 0.001), and VO2 peak on an unrelated admission (r2 = 0.48, P = 0.012) (Figure 2). R2 = 0.83 P < 0.001 4 2 3 4 5 6 7 8 9 log(30-min Exercise GH AUC) 8 7 6 5 R2 = 0.63 P = 0.001 4 -3 -2 -1 0 1 2 3 4 log(Peak Exercise GH) R2 = 0.48 P = 0.012 4 5 10 15 20 25 30 35 40 45 50 VO2 Peak Figure 1. Univariate Relationships between loge(24-Hr GH AUC) and: Subject Age, BMI, loge(30-Min Exercise GH AUC), loge(Peak Exercise GH) and VO2 Peak , Within the Female Subgroup. R2 denotes the proportion of the total variation in the loge(24-hr GH AUC) values that is explained by the predictor variable values, and P denotes the P-value for the test of the null hypothesis that there is no association between the log e(24-hr GH AUC) values and the predictor variable values. In males, the multivariate regression analysis revealed that significant variability in NES log e(24-hr IGHC) was explained by subject age and BMI together (P = 0.020), but not by either of the two variables alone (P = 0.147 and P = 0.078, respectively) after accounting for the variability in NES 24hr IGHC that was explained by the remaining set of predictor variables. Neither the GH response to exercise (30-min of exercise-induced IGHC, peak exercise-induced GH concentration) or the fitness level (i.e., VO2 peak) explained significant unique variability in NES loge(24-hr GH IGHC) (P = 0.958, P = 0.699, and P = 0.725, respectively) (refer to Table 3). Individually, none of the regression model predictor variables was uniquely associated with NES loge(24-hr IGHC) (Table 3), which in all likelihood is the consequence of the high degree of correlation between several of the predictor variables in males (Table 4). 50 Table 3. Multivariate Regression Model for Examining Partial Associations between NES 24-Hr IGHC and Age, BMI, Exercise-Induced IGHC during 30 Min of Exercise, Peak Exercise-Induced GH Concentration and VO2 Peak in Males. 95% Source Parameter Standard Confidence F P-value P-value† Of Variation Estimate Error Interval Statistic* Intercept 11.65 2.71 [5.61, 17.68] Age -0.06 0.04 [-0.14, 0.02] 2.47 0.147 } 0.020 BMI -0.09 0.05 [-0.20, 0.01] 3.84 0.078 loge(30-Min IGHC)‡ -0.02 0.41 [-0.93, 0.89] 0.00 0.958 } 0.750 loge(Peak GH)§ 0.12 0.30 [-0.54, 0.78] 0.16 0.699 VO2 Peak -0.01 0.03 [-0.09, 0.06] 0.13 0.725 Model 4.00 0.030 2 R adjusted║ 0.50 *Type III F-statistic for testing the null hypothesis that the “source of variation” is not uniquely associated with loge(NES 24-hr IGHC) after taking into account the variation explained by the remaining sources of variation. †P-value of the tests of the null hypothesis that the regression coefficients associated with the two sources of variation are both equal to zero. ‡The natural log transformed values of exercise-induced IGHC during 30 min of exercise. §The natural log transformed values of peak exercise-induced GH concentration. ║Adjusted coefficient of multiple determination. Table 4. Spearman Correlations between the Individual Predictor Variables of NES 24-Hr IGHC in Males. Predictor Variable Predictor Variable BMI loge(30-Min IGHC)* loge(Peak GH)† VO2 Peak Age 0.47 -0.35 -0.33 0.15 0.070 0.180 0.220 0.580 p‡ BMI -0.63 -0.61 -0.55 0.010 0.010 0.030 p loge(30-Min IGHC)* 0.93 0.44 <0.001 0.090 p Peak GH 0.40 0.120 p *Natural log transformed values of exercise-induced IGHC during 30 min of exercise. †Natural log transformed values of peak exercise-induced GH concentration. ‡Denotes the P-value for the test of the null hypothesis that the correlation between the ordered ranks of the two predictor variables is equal to zero. In the females, multivariate regression analyses revealed that significant unique variability in NES loge(24-hr GH IGHC) was explained by the subjects’ age (P = 0.048). The variability in NES loge(24hr IGHC) explained by the GH response to exercise (30 min of exercise-induced IGHC and peak exercise-induced GH concentration) was also significant (P = 0.046), although neither 30 min of exercise-induced IGHC (P = 0.376) or peak exercise-induced GH concentration (P = 0.633) explained unique variability in NES loge(24-hr GH IGHC) by itself after accounting for the variability in NES loge(24-hr GH IGHC) that was explained by the remaining set of predictor variables. Like in males, fitness level (i.e., VO2 peak) was not uniquely associated with NES loge(24-hr GH IGHC). Individually, “subject age” was the only predictor variable that was uniquely associated with NES log e(24-hr GH IGHC) (Table 5), which again in all likelihood is the consequence of the high degree of correlation between several of the predictor variables (Table 6). 51 Table 5. Multivariate Regression Model for Examining Partial Associations between NES 24-Hr IGHC and Age, BMI, Exercise-Induced IGHC during 30 Min of Exercise, Peak Exercise-Induced GH Concentration and VO2 Peak in Females. Source Of Variation Intercept Age BMI loge(30-Min IGHC)‡ loge(Peak GH)§ VO2 Peak Model R2 adjusted║ Parameter Estimate 5.49 -0.04 -0.01 0.33 0.20 0.03 Standard Error 1.73 0.02 0.03 0.34 0.39 0.02 95% Confidence Interval [1.25, 9.73] [-0.08, 0.00] [-0.09, 0.08] [-0.51, 1.17] [-0.76, 1.15] [0.02, 0.07] F P-value Statistic* 6.17 0.02 0.91 0.25 1.95 13.48 0.048 0.881 0.376 0.633 0.212 0.003 P-value† } 0.167 } 0.046 0.85 *Type III F-statistic for testing the null hypothesis that the “source of variation” is not uniquely associated with log e(NES 24-hr IGHC) after taking into account the variation explained by the remaining sources of variation. †P-value of the tests of the null hypothesis that the regression coefficients associated with the two sources of variation are both equal to zero. ‡The natural log transformed values of exercise-induced IGHC during 30 min of exercise. §The natural log transformed values of peak exercise-induced GH concentration. ║Adjusted coefficient of multiple determination. Table 6. Spearman Correlations between the Individual Predictor Variables of NES 24-Hr IGHC in Females. Predictor Variable Predictor Variable BMI loge(30-Min IGHC)* loge(Peak GH)† VO2 Peak Age 0.10 -0.13 0.07 0.14 0.730 0.670 0.820 0.670 p‡ BMI -0.66 -0.64 -0.89 0.010 0.020 <0.001 p loge(30-Min IGHC)* 0.92 0.66 <0.001 0.020 p Peak GH 0.58 0.050 p *Natural log transformed values of exercise-induced IGHC during 30 min of exercise. †Natural log transformed values of peak exercise-induced GH concentration. ‡Denotes the P-value for the test of the null hypothesis that the correlation between the ordered ranks of the two predictor variables is equal to zero. DISCUSSION The findings of the present study highlight the gender-mediate differences in the relationship between exercise-induced GH release and spontaneous, non-stimulated GH release on a separate day. Our data demonstrate that the IGHC from a 30-min bout of exercise can predict total non-stimulated 24-hr IGHC on a separate, unrelated day in females only. This information should help promote new insight into program design (i.e., diet, exercise, and healthy living habits) for females by clinicians, dieticians, and exercise physiologists. Clearly, the data indicate that exercise is more highly correlated to GH output in females versus males and, therefore, exercise interventions should be the primary focus for achieving optimal health and well-being in females. 52 While the results are similar to previously reported observations that the GH released during rest and exercise is independently influenced by gender, age, BMI, and physical fitness status, the present study appears to be the first to report gender differences in the joint effects of descriptive predictor variables (age, gender, BMI, and fitness level) in conjunction with the exercise-induced GH response on total 24-hr non-exercise IGHC. Overall, our multiple regression models revealed that 92% of the variance in predicting 24-hr GH IGHC could be explained from age, BMI, fitness level, and 30 min of exercise-induced IGHC in the females compared to 71% in the males. Although the overall regression models explained a significant amount of total variance in both genders, our results provide new evidence of the unique predictor variable differences between males and females. The primary purpose of the present study was to test the hypothesis that the exercise-induced GH response during an acute 30-min constant load exercise is associated with total non-stimulated 24-hr GH concentration on a separate independent day, regardless of gender, age, BMI, or VO2 peak. However, the present findings in the present study indicate that the exercise-induced GH response is associated with and may be used to predict non-stimulated 24-hr IGHC in females but not in males. This finding confirms the gender disparity in the exercise-induced GH response that has been noted in many studies using a variety of exercise modalities, protocols, and measurement techniques. Thus, the finding has practical implications for clinical research and testing especially in regards to exercise interventions aimed at improving total GH output in females. Equating Workload between Genders This study used an exercise stimulus that was relative for each subject (power output set midway between the pre-determined VO2 at LT and VO2 peak), yet similar in intensity to previous research. Despite equating workload intensity between males and females, females had ~1.7 times the amount of exercise-induced IGHC and ~1.6 fold higher exercise-induced peak GH than males during the 30min constant load exercise bout. There were no statistically significant gender differences in total exercise-induced GH IGHC during the 30-min constant load exercise (P = 0.131), the peak GH during exercise (P = 0.089), or the unrelated non-exercise 24-hr IGHC (P = 0.488), but results from the multivariate regression analysis showed that the exercise-induced GH response still represented a greater portion of the total 24-hr GH output on a separate, unrelated non-exercise 24-hr occasion in females versus males. Total GH concentration during 30 min of constant load exercise accounted for ~31.8% of the total 24-hr GH concentration in females compared to ~23.7% in males. Therefore, total non-exercise 24-hr GH concentration can be adequately predicted by the exercise-induced GH response in as little as 30 min of exercise on a separate, unrelated occasion in females only (Table 5). Exercise-Induced Growth Hormone “Snapshot” Even though 30 min is 2% of a 24-hr period, a substantial amount of GH was released during that short time frame (specifically, 23.7% in males and 31.8% in females). Therefore, when adding additional recovery time (330 min) that would explain 27% of a 24-hr period, the total GH concentration released increased minimally to 28.4% and 36.1% in males and females, respectively of the total 24-hr GH concentration. Based on negligible 5% differences in GH concentration and no associated changes in the statistical model when more post-exercise recovery time was included in the analysis, we conclude that minimal additional information related specifically to the total 24-hr GH concentration is gleaned from collecting GH profiles during recovery from exercise. However, monitoring GH concentration during the post-exercise recovery period may also provide valuable information about other incremental GH secretion variables (e.g., time to return to basal secretion, GH half-life, etc.). 53 Fitness Level and Growth Hormone Release Additional parameters known to concomitantly influence total GH concentration were examined in an attempt to further explain the association between exercise-induced and total non-exercise 24-hr GH concentration. Peak oxygen consumption (VO2 peak) was significantly related to non-exercise 24-hr IGHC in females (Figure 2). But, when using the multivariate regression analysis to examine the covariate adjusted relationship, VO2 peak was no longer uniquely associated with total non-exercise 24-hr IGHC for either gender. Weltman et al. (24) previously found that fitness level (as assessed by VO2 peak) was significantly correlated with total 24-hr GH concentration in males but not females. Although the subjects used in this study are slightly more overweight and less fit, further examination of the regression line from Figure 1d of Weltman et al. (24) suggests that at lower VO2 peak values (such as <40 mL·kg-1·min-1) the relationship between VO2 peak and total 24-hr GH concentration is stronger in females than males. If this interpretation is accurate, then further work needs to be done across broader ranges of exercise capacity to determine if VO 2 peak is truly a significant predictor of 24-hr GH release. This also suggests that VO2 peak is not the only determinant of exercise capacity and our data on the “snapshot” of 30 min of constant load total exercise-induced GH concentration may provide unique input on total 24-hr GH concentration that is especially true in females. BMI, Gender, and Growth Hormone Release In a previous study, total 24-hr GH concentration was simultaneously determined by BMI and gender, while peak GH values were significantly influenced by BMI in both males and females (23). In the current study, multiple regression analysis revealed that significant variability in non-exercise 24-hr IGHC was explained by age and BMI in males but not independently after accounting for the variability that was explained by the other predictor variables (Table 3). However, in females, only age was uniquely associated with non-exercise 24-hr IGHC (Table 5). Although the range of BMI in the current study was large and could have influenced the results of the statistical model, it was similar to the BMI range reported by Veldhuis et al. (23). While they also reported BMI to have a significant negative relationship to total GH concentration across both genders, it was only significant when jointly associated with gender and IGF-1 simultaneously. An examination of the results of Weltman et al. (24) suggest that: (a) at lower BMI levels the relationship between BMI and GH concentration appears to be more strongly associated in the males compared to the females; and (b) at higher BMI levels the relationship between BMI and GH concentration may become stronger in the females. In obese individuals, many of the beneficial effects of GH are absent, regardless of gender or age in this population (9,22). The lack of a relationship between BMI and GH concentration in females using the multivariate statistical approach is unexpected, considering GH has a strong lipolytic effect (11,13) and women typically release greater amounts of GH. Clasey et al. (1) conducted a large-scale study on physiological factors known to alter 24-hr GH secretion. They concluded that abdominal visceral fat was a stronger predictor of 24-hr pulsatile GH secretion than BMI, regardless of age or gender. An individual’s BMI does not distinguish between lean and fat mass and phenotypic differences in body composition known to be gender-mediated. In general, females accumulate more subcutaneous fat than visceral fat compared to males and males have more lean muscle mass in their arms while females tend to accumulate more of their fat mass in the lower body (29). However, it is not known whether total exercise-induced GH concentration may be influenced by gender differences in subcutaneous versus intra-visceral fat reserves during exercise. Regardless, the biological activity of GH indicates that exercise may be an important precursor to beneficial changes in lean body mass and body fat. The GH has been shown to augment adipose tissue lipolysis and, therefore, exercise-induced GH release may be a good therapeutic agent for obtaining optimal body composition (especially in females). 54 Practical Laboratory Testing Implications Laboratory testing procedures to determine total 24-hr IGHC are time consuming and costly and also require the use of a highly sensitive assay (16). Stimulation tests can report the level of GH in the blood after the infusion of GH stimulants (i.e., insulin, glucagon, arginine or GH-releasing hormone) that measure the ability of the pituitary gland to release GH. Even though these tests can be done in shorter time frames than 24 hrs, these tests can still take several hours. Given the significant participant and laboratory burden related to the collection of time-lengthened GH profiles to examine 24-hr GH output, exercise-induced GH results could provide researchers with the capability of using a brief exercise-induced snapshot of the GH response to estimate total 24-hr GH concentration in females without having to conduct extensive 24-hr clinical testing. Limitations Our statistical regression model used 16 males with 2 predictors, which gave an 8:1 subject-topredictor. Our 14 females to 3 predictor ratio is less than the suggested 10:1 subject-to-predictor ratio for running continuous variables. Therefore, we recognize the slight limitation of statistical power with our methodology. Additionally, the low fitness level of our subjects (10th percentile) means we can only infer our results on the “un-fit” population and more fit subjects may not respond in exactly the same manner. Since age has a unique association with GH release, our results also cannot be interpreted for older adult populations. Regardless of the possibility of over-fitting our data and our focused demographics, the exact mechanisms causing gender differences in the relationship between the exercise-induced GH response during an acute 30-min bout of exercise and total 24-hr GH concentration are unknown and our results provide valuable information for the young adult to middle-aged “un-fit” population. CONCLUSIONS To our knowledge, this is the first study to use constant load exercise-induced GH concentration in a multivariate regression analysis to predict total non-exercise 24-hr GH output on a separate occasion in human subjects. While exercise-induced GH concentration during constant load exercise (~74 VO2 peak) provided important information on total non-exercise 24-hr IGHC in females on a separate occasion, it had no relationship to total non-exercise 24-hr GH concentration in males. Thus, we conclude that total exercise-induced GH concentration is a stronger predictor of total non-stimulated 24-hr GH concentration in females and those interventions for increasing total 24-hr GH concentration should be aimed at increasing exercise habits in females. Additionally, GH release during a 30-min constant load exercise can provide valuable insight into total non-exercise 24-hr GH output in females on a separate occasion without extensive 24-hr blood sampling. Gender disparities in GH release are well documented at rest, but less is known about the gender differences in exercise-induced GH concentration and how these differences may influence total 24-hr GH concentration. While many GH stimulation tests are available for the diagnosis of GH deficiencies, the exercise-induced GH response to constant load exercise may provide additional insights into clinically relevant subtle gender differences in GH release. 55 ACKNOWLEDGMENTS We acknowledge the nursing staff of the General Clinical Research Center (GCRC) for drawing blood and caring for patients and the members of the Core Laboratory for performing the assays. Address for correspondence: Kevin Ritsche, PhD, Department of Exercise Physiology, WinstonSalem State University, Winston-Salem, NC, USA 27110, Email: ritschek@wssu.edu REFERENCES 1. Clasey JL, Weltman A, Patrie J, Weltman JY, Pezzola S, Bouchard C, Thorner MO, Hartman ML. Abdominal visceral fat and fasting insulin are important predictors of 24-hour GH release independent of age, gender, and other physiological factors. J Clin Endocrinol Metab. 2001; 86:3845-3852. 2. Djurhuss CB, Gravholt CH, Nielsen S, Pedersen SB, Moller N, Schmitz O. Additive effects of cortisol and growth hormone on regional and systemic lipolysis in humans. Am J Physiol. 2004;286:EE488-EE494. 3. Doessing S, Heinemeier KM, Holm L, Mackey AL, Schjerling P, Rennie M, Smith K, Reitelseder S, Kappelgaard AM, Rasmussen NH, Flyvbjerg A, Kjaer M. Growth hormone stimulates the collagen synthesis in human tendon and skeletal muscle without affecting myofibrillar protein synthesis. J Physiol. 2010;588:341-351. 4. Ho KY, Evans WS, Blizzard RM, Veldhuis JD, Merriam GR, Samojilk E, Furlanetto R, Rogol AD, Kaiser DL, Thorner MO. Effects of sex and age on the 24-hour profile of growth hormone secretion in man: Importance of endogenous estradiol concentrations. J Clin Endocrinol Metab. 1987;64:51-58. 5. Irving BA, Weltman JY, Patrie JT, Davis Ck, Brock DW, Swift D, Barett EJ, Gaesser GA, Weltman A. Effects of exercise training intensity on nocturnal growth hormone secretion in obese adults with the metabolic syndrome. J Clin Endocrinol Metab. 2009;94:1979-1986. 6. Kanaley JA, Weatherup-Dentes MM, Jaynes EB, Hartman ML. Obesity attenuates the growth hormone response to exercise. J Clin Endocrinol Meta. 1999;84:3156-3161. 7. Kanaley JA, Weltman JY, Velduis JD, Rogol AD, Hartman ML, Weltman A. Human growth hormone response to repeated bouts of aerobic exercise. J Appl Physiol. 1997;1756-1761. 8. Kindermann W, Schnabel A, Schmitt WM, Biro G, Cassens J, Weber F. Catecholamines, growth hormone, cortisol, insulin, and sex hormones in anaerobic and aerobic exercise. Eur J Appl Physiol. 1982;49:389-399. 9. Lin CM, Huang YL, Lin ZY. Influence of gender on serum growth hormone, insulin-like growth factor-I and its binding protein-3 during aging. Yonsei Med J. 2009;50:407-413. 56 10. Makimura H, Stanley T, Mun D, You SM, Grinspoon S. The effects of central adiposity on growth hormone (GH) response to GH-releasing hormone-arginine stimulation testing in men. J Clin Endocrinol Metab. 2008 93:4254-4260. 11. Nass R, Thorner MO. Impact of GH-cortisol ratio on the age-dependent changes in body composition. Growth Horm IGF Res. 2002;12:147-161 12. Nindl BC, Scoville CR, Sheehan KM, Leone CD, Mello RP. Gender differences in regional body composition and somatotrophic influences of IGF-1 and leptin. J Appl Physiol. 2002;92: 1611-1618. 13. Prifzlaff CJ, Wideman L, Blumer J, Jensen M, Abbott RD, Gaesser GA, Veldhuis JD, Weltman A. Catecholamine release, growth hormone secretion, and energy expenditure during exercise vs. recovery in men. J Appl Physiol. 2000;89:937-946. 14. Pritzlaff CJ, Wideman L, Weltman JY, Abbott R, Gutgesell M, Hartman ML, Veldhuis JD, Weltman A. Gender governs the relationship between exercise intensity and growth hormone release in young adults. J Appl Physiol. 2002;92:2053-2060. 15. Quabbe HJ, Bratzke HJ, Siegers U, Elban K. Studies on the relationship between plasma free fatty acids and growth hormone secretion in man. J Clin Invest. 1972;51:2388-2398. 16. Reutens AT, Hoffman DM, Leung KC, Ho KY. Evaluation and application of a highly sensitive assay for serum growth hormone (GH) in the study of adult GH deficiency. J Clin Endocrinol Metab. 1995;80(2):480-485. 17. Salvadori A, Fanari P, Marzullo P, Codecase F, Tovaglieri L, Cornacchia M, Walker G, Brunani A, Longhini E. Dynamics of GH secretion during incremental exercise in obesity, before and after a short period of training at different work-loads. Clin Endocrinol. 2010;73:491-496. 18. Sartorio A, Agosti F, Marinone PG, Proietti M, Lafortuna CL, Maffluletti NA. Growth hormone responses to repeated bouts of aerobic exercise with different recovery intervals. J Appl Physiol. 2006;100:1093-1094. 19. Stokes KA, Nevill ME, Lakomy HK, Hall GM. Reproducibility of the growth hormone response to sprint exercise. Growth Horm IGF Res. 2003;13:336-340. 20. Vahl N, Jergensen JO, Skjaerbaek C, Veldhuis JD, Orskov H, Christiansen JS. Abdominal adiposity rather than age and sex predicts mass and regularity of GH secretion in healthy adults. Am J Physiol. 1997;272(6):E1108-1116. 21. Veldhuis JD, Johnson ML. Cluster analysis: A simple, versatile, and robust algorithim for endocrine pulse detection. Am J Physiol. 1986;250:E486-E493. 22. Veldhuis JD, Patrie JT, Brill KT, Weltman JY, Mueller EE, Bowers CY, Weltman A. Contributions of gender and systemic estradiol and testosterone concentrations to maximal secretagogue drive of burst-like growth hormone secretion in healthy middle-aged and older adults. J Clin Endocrinol Metab. 2004;89:6291-6296. 57 23. Veldhuis JD, Roelfsema F, Keenan DM, Pincus S. Gender, age, body mass index, and IGF-1 individually and jointly determine distinct GH dynamics: Analyses in one hundred healthy adults. J Clin Endocinrol Metab. 2011;96:115-121. 24. Weltman A, Weltman YJ, Hartmann ML, Abbott RD, Rogol AD, Evans WS, Veldhuis JD. Relationship between age, percentage body fat, fitness, and 24-hour growth hormone release in healthy young adults: Effects of gender. J Clin Endocrinol Metab. 1994;78(3):543-548. 25. Weltman A, Weltman YJ, Waston-Winfield DD, Frick K, Patrie J, Kok P, Keenan DM, Gaesser GA, Veldhuis JD. Effects of continuous versus intermittent exercise, obesity, and gender on growth hormone secretion. J Clin Endocrinol Metab. 2008;93:4711-4720. 26. Wideman L, Consitt L, Patrie J, Swearingin B, Bloomer R, Davis P, Weltman A. The impact of sex and exercise duration on growth hormone secretion. J Appl Physiol. 2006;101:16411647. 27. Wideman L, Weltman Y, Patrie J, Bowers CY, Shah N, Story S, Weltman A, Veldhuis JD. Synergy of L-arginine and growth hormone (GH)-releasing peptide-2 on GH release: Influence of gender. Am J Physiol. 2000;279:R1455-1466. 28. Wideman L, Weltman JY, Shah N, Story S, Veldhuis JD, Weltman A. Effects of gender on exercise-induced growth hormone release. J Appl Physiol. 1999;87:1154-1162. 29. Wirth A, Steinmetz B. Gender differences in changes in subcutaneous and intra-abdominal fat during weight reduction: An ultrasound study. Obes Res. 1998;6:393-399. 30. Zhao JY, Cowley MJ, Lee P, Birzniece V, Kaplan W, Ho KK. Identification of novel GHregulated pathway lipid metabolism in adipose tissue: A gene expression study in hypopituitary men. J Clin Endocrinol Metab. 2011;96:1188-1196. Disclaimer The opinions expressed in JEPonline are those of the authors and are not attributable to JEPonline, the editorial staff or the ASEP organization.