CHM 115: GENERAL Chemistry I (4.0 Credits) TENTATIVE CLASS
advertisement

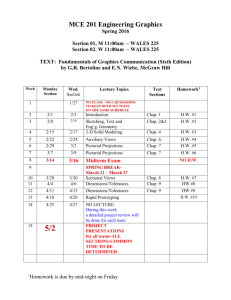
CHM 115: GENERAL CHEMISTRY I (4.0 Credits) TENTATIVE CLASS SCHEDULE Fall Semester 2009 / CRN 30457, 30459 & 30462 Unit Dates Activities Unit 1 Week 1 Aug. 31 (M) & Sep. 2 (W) Scope/Reading: Course Syllabus, Supplementary Reading 1, and Silberberg Chap. 1 and Chap. 2 on Physical and Chemical Changes Lab/SI Period: Review names and symbols of elements; review metric and SI units; review ratio and dimensional analysis (with metric and SI units); review logarithm (common and natural); review linear equations and slope Week 2 Sep. 9 (W) Scope/Reading: Silberberg Sec. 6.1, 6.2, 6.4, 16.1, 16.8, and 20.4 on Reaction Enthalpy, Spontaneity, Speed, and Catalysis *Sep. 7 (M) is Labor Day Recess. Lab/SI Period: Review names and formulas of compounds; review first and second laws of thermodynamics; review the equilibrium concept; review the spontaneity concept; review the factors that affect the speed of a chemical reaction; complete the check-in procedure (in lab); complete the lab safety and successful lab report discussions (in lab); complete Lab. 01 Week 3 Sep. 14 (M) & Sep. 16 (W) Scope/Reading: Silberberg Chap. 3 and Chap. 5 on the Kinetic Molecular Theory, Collision Theory, and the Use of Chemical Equations Lab/SI Period: Review the mole concept and molar quantities; review dimensional analysis involving molar quantities; review molarity as a molar quantity; review balancing chemical equations; review reaction stoichiometry; review theoretical and actual yield calculations; complete Lab. 02 Week 4 Sep. 21 (M) & Sep. 23 (W) Scope/Reading: Silberberg Chap. 4 on the Major Classes of Aqueous-phase Reactions Lab/SI Period: Review the composition of a solution; review solubility; review molecular and net ionic equations; review assignment of oxidation numbers; review oxidation, reduction, oxidizing agent, and reducing agent concepts; review the thermodynamics and kinetics of precipitation, acid-base, and oxidation-reduction reactions; review for the FIRST EXAM; complete Lab. 03 FIRST EXAM: Sep. 28 (M), 9:30-10:50 a.m. (covering everything discussed in Unit 1 from Week 1 to Week 4) Purdue University Calumet -1- CHM 115, Fall 2009 Unit Dates Activities Unit 2 Week 5 Sep. 30 (W) Scope/Reading: Silberberg Sec. 6.3, 6.5, and 6.6 on Calorimetry and Hess’s Law Lab/SI Period: Review the state function concept; review the standard state concept; review Hess’s law calculations; complete Lab. 04 Week 6 Oct. 5 (M) & Oct. 7 (W) Scope/Reading: Supplementary Reading 2 & Silberberg Chap. 7 and Chap. 8 on Dalton’s Atomic Theory (ca. 1808) and the Development of Quantum Mechanics (through pretty much all of the 20th century) Lab/SI Period: Review wave mechanics; review the wave-particle duality; review the Heisenberg Uncertainty Principle; review electron spin and Hund’s Rule; review quantum numbers; review atomic orbitals; review electron configurations for elements; complete Lab. 05 Week 7 Oct. 12 (M) & Oct. 14 (W) Scope/Reading: Continue with Silberberg Chap. 8 on The Periodicity of the Chemical Elements Lab/SI Period: Review the properties of the main group and transition elements; review electron configurations for monatomic cations and anions; review the periodic trends in atomic & ionic sizes and ionization energies; complete Lab. 06 Week 8 Oct. 19 (M) & Oct. 21 (W) Scope/Reading: Begin Silberberg Chap. 9 and Chap. 11 on Chemical Bonding Lab/SI Period: Review for the SECOND EXAM; complete Lab. 07 SECOND EXAM: Oct. 26 (M), 9:30-10:50 a.m. (covering everything discussed in Unit 2 from Week 5 to Week 8) Unit Dates Activities Unit 3 Week 9 Oct. 28 (W) Scope/Reading: Continue with Silberberg Chap. 9 and Chap. 11 on Chemical Bonding Lab/SI Period: Review the Lennard-Jones (6-12) potential; review the electronegativity concept and the periodic trend in electronegativity; review molecular orbital (MO) diagrams for simple homo- and heteronuclear diatomic molecules; complete Lab. 08 Week 10 Nov. 2 (M) & Nov. 4 (W) Scope/Reading: Conclude Silberberg Chap. 9 and Chap. 11 and begin Chap. 10 on Chemical Bonding and the Lewis Model and Lewis Structures Lab/SI Period: Review the drawing of Lewis structures; review the resonance concept; review the VSEPR theory; review molecular polarity; review hybrid orbitals and the Valence Bond (VB) Theory; complete Lab. 09 Purdue University Calumet -2- CHM 115, Fall 2009 Unit Dates Activities Unit 3 (Cont.) Week 11 Nov. 9 (M) & Nov. 11 (W) Scope/Reading: Continue with Silberberg Chap. 10 and Sec. 12.3, 15.1, 15.2, 23.4, and 23.5 on Molecular Structures and Properties Lab/SI Period: Review intermolecular forces; review hydrocarbons; review isomerism; review stereochemistry; review coordination compounds; review the Crystal Field Theory; complete Lab. 10 Week 12 Nov. 16 (M) & Nov. 18 (W) *Nov. 20 (F) is the last day to drop Fall 2009 classes. Scope/Reading: Supplementary Reading 3 and Sec. 12.6, 15.5, and 15.6 on Structures and Properties of Solids and Polymers Lab/SI Period: Review x-ray crystallography; review the unit cell concept and calculations; review the band theory of conductivity; review solar cells and superconductivity; review the polymerization concept; review for the THIRD EXAM; complete Lab. 11 THIRD EXAM: Nov. 23 (M), 9:30-10:50 a.m. (covering everything discussed in Unit 3 from Week 9 to Week 12) *No CHM 115 lecture and lab will take place between Nov. 24 (Tu) and Nov. 27 (F) due to Fall Recess and Thanksgiving holidays. However, Nov. 24 (Tu) is NOT an actual holiday. Activities outside of CHM 115 lecture and lab should take place as scheduled unless you are being notified otherwise. Unit Dates Activities Unit 4 Week 14 Nov. 30 (M) & Dec. 2 (W) Scope/Reading: Silberberg Chap. 24 on Nuclear Chemistry Week 15 Dec. 7 (M) & Dec. 9 (W) Scope/Reading: “Review Week” – Review everything from Week 1 to Week 14 Week 16 Dec. 14 (M) Comprehensive FINAL EXAM 8:00-10:00 a.m. Final Exam Purdue University Calumet Lab/SI Period: Review radiation; review the pathways for nuclear decay; review applications of radioactivity; review the binding energy concept; review fission and fusion and their applications; review first-order kinetics; review the first-order integrated rate law; review half-life; review C-14 dating; complete Lab. 12 Lab/SI Period: Review for the FINAL EXAM; complete the check-out procedure (in lab); complete the National (ACS) First-semester General Chemistry Exam (in lab); complete Course and Lab Instructor Evaluations (in lab) -3- CHM 115, Fall 2009