Spectroscopy - Dover College Science
advertisement

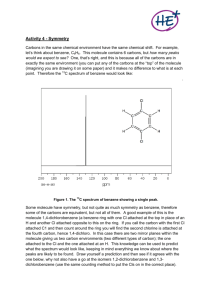
A2 CHEMISTRY NOTES: SPECTROSCOPY Infrared in analysis We studied the use of IR radiation for analytical purposes at AS level. We know that Only ___________ molecules absorb infra-red radiation Absorbing IR radiation causes ________ stretching and bond __________. Different bonds absorb at ____________ wave lengths (wave numbers cm-1) Different bonds have characteristic absorption __________ (weak, medium, strong) Different bonds have characteristic _________ of absorption (broad, sharp) Use the IR table from your data sheet to identify the six IR spectra above. 1. octanoic acid 2. Cyclohexanol 3. squalane, 2,6,10,15,19,23 hexamethyltetracosane, C30H62 4. 4-methylbenzaldehyde 5. diethyl malonate 6. 2-hydroxybenzaldehyde 7. diphenyl methane 1 A2 CHEMISTRY NOTES: SPECTROSCOPY Mass Spectroscopy The mass spectrometer is used in determining the structure of molecules. In a mass spectrometer, molecules are converted to _________ ions; the positive _________ ions formed then break. This is called _______________. M+• → X+ + Y• The fragmentation pattern and the abundance of the fragments is characteristic for individual molecules. Fragmentation occurs to give a greater incidence of the more ______ ions 2°, 3 carbocations and _______, acylium ions. Match the compounds with the mass spectrum below: Pentan-2-one Pentan-3-one Pentane Butan-1-ol Butan-2-ol Phenylamine 2 Ethylamine 2-methylbutane A2 CHEMISTRY NOTES: SPECTROSCOPY What Is Nuclear Magnetic Resonance (Nmr)? Nuclear magnetic resonance is concerned with the magnetic properties of certain nuclei such as ___ and ____. These atoms (nuclei) absorb energy in the radio-wave region of the electromagnetic spectrum. The exact frequency at which they absorb depends on their ____________ (what they are bonded to, what they are near) Features of an H-1 NMR spectrum A simple NMR spectrum looks like this: The peaks There are two peaks because there are two different ___________ for the hydrogens in the CH3 group and attached to the oxygen in the _____ group. They are in different places in the spectrum because they resonate at slightly different _________. The sizes of the two peaks gives important information about the _______ of hydrogen atoms in each ___________. It isn't the height of the peaks that matters, but the ratio of the _______ under the peaks. If you could measure the areas under the peaks in the diagram above, you would find that they were in the ratio of __: __ (__for the larger peak __ for the smaller one). That shows a ratio of ____ in the number of hydrogen atoms in the two environments which is exactly what you would expect for CH3COOH. 3 A2 CHEMISTRY NOTES: SPECTROSCOPY The need for a standard for comparison - TMS Before we can explain what the horizontal scale means, we need to explain the fact that it has a ______ point - at the right-hand end of the scale. The zero is where you would find a peak due to the hydrogen atoms in tetramethylsilane - usually called ____. Everything else is compared with this. TMS is chosen as the standard for several reasons. The most important are: It has 12 hydrogen atoms all of which are in exactly the _____ environment. That produces a _______, strong peak (because there are lots of __________ atoms) and so only a trace amount is needed. It is volatile and can readily be ____________ off to recover the material. TMS produces a peak on the spectrum at the extreme ________-hand side. Almost everything else produces peaks to the _________ of it. It doesn’t usually interact with other molecules and so does not affect the chemical _______ values. The chemical shift The horizontal scale is shown as (ppm). is called the ___________ shift and is measured in parts per million - ____. A peak at a chemical shift of 2.0 is said to be ____________ of TMS. The further to the left a peak is, the more __________ it is. Solvents for NMR spectroscopy NMR spectra are usually measured __________ solvents in which any ordinary hydrogen atoms are replaced by its _________, __________ - for example, CDCl3 instead of _______. As there is usually only a small amount (milligrams) of substrate dissolved in 1cm3 of solvent if the solvent were not ___________ the signals of the substrate would completely _________ by the signals of the solvent. 4 A2 CHEMISTRY NOTES: SPECTROSCOPY Low Resolution Nmr Spectra In a low resolution spectrum the numbers against the peaks represent the relative _____ under each peak. Interpreting a low resolution spectrum Using the total number of peaks Each peak represents a different ____________ for __________ atoms in the molecule. In the methyl propanoate, CH3CH2COOCH3, spectrum above, there are _______ peaks because there are _______ different ____________ for the hydrogens. Using the areas under the peaks The ratio of the areas under the peaks tell you the ratio of the numbers of __________ in the various ____________. In the methyl propanoate case, the areas were in the ratio of ______, which is exactly what you want for the two differently placed CH3 groups and the CH2 group. Using chemical shifts The position of the peaks tells you useful things about what groups the various ________ atoms are in. In any exam, you will be given a table of __________ shifts if you need them. The important shifts for the groups present in methyl propanoate are: 5 A2 CHEMISTRY NOTES: SPECTROSCOPY Some sample questions Example 1 An organic compound was known to be one of the following. Use its low resolution NMR spectrum to decide which it is. Notice that there are ________ peaks showing ____ different ___________ for the hydrogens. That eliminates _________ as a possibility because that would only give ____ peaks - due to the ___ differently situated CH3 group hydrogens. Does the ratio of the areas under the peaks help? Not in this case - both the other compounds would have three peaks in the ratio of ______. Now you need to look at the chemical shifts: Checking the positions of the various hydrogens in the two possible compounds against the chemical shift table gives you this pattern of shifts: Comparing these with the spectrum means that the substance was ______________________,. 6 A2 CHEMISTRY NOTES: SPECTROSCOPY Example 2 How would you use low resolution NMR to distinguish between the isomers propanone and propanal? The propanone would only give ______ peak in its NMR spectrum because both CH3 groups are in an _________ environments - both are attached to -COCH3. The propanal would give ______ peaks with the areas underneath in the ratio ______. You could refer to the chemical shift table above to decide where the peaks are likely to be found, but it isn't necessary. Example 3 How many peaks would there be in the low resolution NMR spectrum of the following compound, and what would be the ratio of the areas under the peaks? All the CH3 groups are in __________ so would produce ___ peak. There would also be peaks for the hydrogens in the CH2 group and the COOH group. There would be _____ peaks in total with areas in the ratio _____. 7 A2 CHEMISTRY NOTES: SPECTROSCOPY HIGH RESOLUTION NMR SPECTRA What a low resolution NMR spectrum tells you Remember: The number of peaks tells you the number of different __________ the hydrogen atoms are in. The _____ of the areas under the peaks tells you the ratio of the numbers of hydrogen atoms in each of these environments. The ___________ shifts give you important information about the sort of environment the hydrogen atoms are in. High resolution NMR spectra In a high resolution spectrum, you find that many of what looked like single peaks in the low resolution spectrum are ______ into clusters of peaks. You will only need to consider these possibilities: 1 peak a singlet 2 peaks in the cluster a doublet 3 peaks in the cluster a triplet 4 peaks in the cluster a quartet You can get exactly the same information from a high resolution spectrum as from a low resolution one - you simply treat each cluster of peaks as if it were a single one in a low resolution spectrum. But in addition, the amount of __________ of the peaks gives you important extra information. Interpreting a high resolution spectrum The n+1 rule The amount of ________ tells you about the number of _________ attached to the carbon atom or atoms next door to the one you are currently interested in. The number of sub-peaks in a cluster is one more than the number of ___________ attached to the next door carbon(s). 8 A2 CHEMISTRY NOTES: SPECTROSCOPY So - on the assumption that there is only one carbon atom with hydrogens on next door to the carbon we're interested in: singlet next door to carbon with __ hydrogens attached doublet next door to a ___ group triplet next door to a ____ group quartet next door to a ____ group Using the n+1 rule What information can you get from this NMR spectrum? Assume that you know that the compound above has the molecular formula C4H8O2. Treating this as a low resolution spectrum to start with, there are ______ clusters of peaks and so _________ different environments for the hydrogens. The hydrogens in those ____ environments are in the ratio ______. Since there are __ hydrogens altogether, this represents a ___ group and two ___ groups. What about the splitting? The CH2 group at about 4.1 ppm is a _________. That tells you that it is next door to a carbon with __ hydrogens attached - a ___ group. The CH3 group at about 1.3 ppm is a ________. That must be next door to a ____ group. This combination of these two clusters of peaks - one a quartet and the other a triplet - is typical of an ethyl group, _______. It is very common. Get to recognise it! Finally, the CH3 group at about 2.0 ppm is a _______. That means that the carbon next door doesn't have any _________ attached. So what is this compound? You would also use chemical shift data to help to identify the environment each group was in, and eventually you would come up with: 9 A2 CHEMISTRY NOTES: SPECTROSCOPY A special case: Alcohols Where is the -O-H peak? This is very confusing! The problem is that the position of the -OH peak varies dramatically depending on the conditions - for example, what solvent is used, the concentration, and the purity of the alcohol - especially on whether or not it is totally _____. A clever way of picking out the -OH peak If you measure an NMR spectrum for an alcohol like ethanol, and then add a few drops of deuterium oxide, D2O, to the solution, allow it to settle and then re-measure the spectrum, the -OH peak ___________! By comparing the two spectra, you can tell immediately which peak was due to the -OH group. The lack of splitting with -OH groups Unless the alcohol is absolutely free of any water, the hydrogen on the -OH group and any hydrogens on the next door carbon don't interact to produce any splitting. The -OH peak is a _________ and you don't have to worry about its effect on the next door hydrogens. Equivalent hydrogen atoms Hydrogen atoms attached to the same carbon atom are said to be _________. Equivalent hydrogen atoms don't cause any ____________ in the spectrum of the other one. But hydrogen atoms on neighbouring carbon atoms can also be __________ if they are in exactly the same environment. For example: These ___ hydrogens are all exactly equivalent. You would get a ___________ with no splitting at all. The molecule now contains different atoms at each end, the hydrogens are no longer all in the same ___________. This compound would give ____ separate peaks on a NMR spectrum. The high resolution spectrum would show that both peaks subdivided into _________ - because each is next door to a differently placed _____ group. 10 A2 CHEMISTRY NOTES: SPECTROSCOPY For each of the compounds A through F indicate the number of distinct groups of equivalent hydrogens. A Number of distinct hydrogen groups: B Number of distinct hydrogen groups: C Number of distinct hydrogen groups: D Number of distinct hydrogen groups: E Number of distinct hydrogen groups: F Number of distinct hydrogen groups: Nmr Spectra - The Integrator Trace What an integrator trace looks like An integrator trace is a computer generated line which is superimposed on an NMR spectrum. In the diagram, the integrator trace is shown in red. What an integrator trace shows An integrator trace measures the relative ______ under the various peaks in the spectrum. The height gained is proportional to the ____ under the peak or group of peaks. You measure the height gained at each peak or group of peaks by measuring the distances shown in green in the diagram above - and then find their _______. For example, if the heights were 0.7 cm, 1.4 cm and 2.1 cm, the ratio of the peak areas would be ______. That in turn shows that the ratio of the _____ atoms in the ___ different environments is ______. 11 A2 CHEMISTRY NOTES: SPECTROSCOPY Nmr Spectra Problems Draw display formula for the following compounds and identify the NMR spectrum for each of them: 2-heptanone Methyl propanoate 2-pentanone 3-methyl propan-1-ol Ethyl propanoate 12 Pentanal 4-methyl propan-2-ol A2 CHEMISTRY NOTES: SPECTROSCOPY 13 A2 CHEMISTRY NOTES: SPECTROSCOPY 14 A2 CHEMISTRY NOTES: SPECTROSCOPY Features of a C-13 NMR spectrum The C-13 NMR spectra are simpler than H-1 spectra. Each C in a different environment give rise to a peak in the C-13 spectrum. If there is more than 1 carbon in an exactly equivalent environment than they will be represented by a single line. The C-13 NMR spectrum for ethanol There are ___ peaks because there are ___ different environments for the carbons. The carbon in the CH3 group is attached to 3 hydrogens and a carbon. The carbon in the CH2 group is attached to 2 hydrogens, a carbon and an oxygen. The C-13 NMR spectrum for a more complicated compound This is the C-13 NMR spectrum for 1-methylethyl propanoate. This time there are ___ lines in the spectrum. That means that there must be ___ different environments for the carbon atoms in the compound. Is that reasonable from the structure? If two (or more) carbon atoms in a compound have exactly the same ________, only a single peak appears in the C-13 spectrum. 15 A2 CHEMISTRY NOTES: SPECTROSCOPY COLUMN CHROMATOGRAPHY Column chromatography is often used to purify compounds made in the lab. It is a process developed by a Russian botanist to separate the ________ mixtures in plants. In thin layer chromatography, the ___________ phase is a thin layer of silica gel or alumina on a glass, metal or plastic plate. Column chromatography works on a much larger scale by packing the same materials into a vertical glass column. A mixture looks green but is in fact a mixture of two _______ compounds - one _____, one _____.. This is loaded onto a column. The tap is opened so that the __________ (the ________ phase) can flow through the column, collecting it in a flask at the bottom. As the solvent runs through, you keep adding fresh ________ to the top so that the column never dries out. This diagram shows what might happen over time. Explaining what is happening The blue compound is more _______ than the yellow one - you can tell this because the blue compound doesn't travel through the column very ________. That means that it must ________ more strongly to the silica gel or alumina than the yellow one. The less ______ yellow one spends more of its time in the solvent and therefore washes through the column much ___________. 16 A2 CHEMISTRY NOTES: SPECTROSCOPY Gas-Liquid Chromatography As in all forms of chromatography gasliquid chromatography involves a ___________ phase (in this gas a high boiling point liquids absorbed onto a solid) and a ________ phase, a gas such as helium. How fast a particular compound travels through the machine will depend on how much of its time is spent moving with the gas as opposed to being attached to the _______ in some way. Very small quantities of the sample that you are trying to analyse are injected into the machine using a small syringe. The injector is contained in an oven whose temperature can be controlled. It is hot enough so that all the sample boils and is carried into the column as a gas by the helium (or other carrier gas). How separation works on the column The column is a long thin tube packed with the _________ phase usually a waxy polymer. The stationery phase is coated with a high boiling _______. Some molecules may dissolve in the liquid ____________ phase. The more soluble molecules will spend more of their time absorbed into the _____________ phase; the less soluble ones will spend more of their time in the ____. Retention time The _____ taken for a particular compound to travel through the column to the detector is known as its ____________ time. This time is measured from the time at which the sample is ___________ to the point at which the display shows a ___________ peak height for that compound. Different compounds have different retention times. High solubility in the liquid phase means a _____ retention time. The detector There are several different types of detector in use. The _______ ionisation detector and ______ spectrometers are both commonly used. 17