Boyle's law and Charles' Law
advertisement

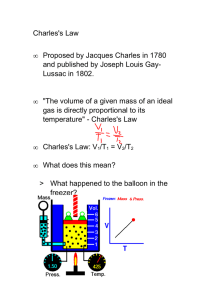
Boyle’s Law and Charles’ Law Kyle Baseden Chemistry I Boyle’s and Charles’ Law 2/22 CCSS.MATH.CONTENT.HSA.SSE.A.1.A-Interpret parts of an expression, such as terms, factors, and coefficients. 1. Materials 1 250 mL flask water empty soda cans Bunsen burner Flint striker Glass dish ice tongs tube for Bunsen burner 2. Objective(s) Calculate the change in either the volume or pressure of a gas, when the amount and temperature of a gas are kept constant. Calculate the change in either the volume or temperature of a gas, when the amount and pressure of a gas are kept constant. Distinguish between the two gas laws. Use the correct law to solve the problem. Be able to apply pressure conversions taught previously to solve problems involving pressure at different units. 3. Motivation If extra review is necessary, stations can be employed o Students can work in their lab groups and move around the room solving problems over pressure conversions. o It can be determined whether this review is necessary based on the evaluations done by the instructor informally in the previous lesson. Engagement o Can crusher At the beginning of the lesson, students will see a can being heated up with a small amount of water inside the can. The can will then be crushed by atmospheric pressure. To crush the can o Obtain an empty soda can, preferably a diet can Diet cans work better because the cans are made with thinner walls. o Fill the soda can with a small amount (about 10-15 mL of water) o Using tongs hold the can with water over a lit Bunsen burner and heat until steam is released from the can. o Upon boiling the water in the can, Revised 4/11 The can should be removed from the flame and immediately inverted into a dish containing ice water. The can should be immediately crushed by the pressure of the air around the can. The instructor should ask the students if they have any idea how this could happen using any knowledge they have. The reason this happens is that the volume of the gas decreases as the temperature of the water vapor decreases. This reduces the pressure the gas enacts on the interior of the can. o As the pressure of the gas decreases inside the can, the pressure outside the can due to the pressure from the air remains constant. The pressure outside the can will be much higher than the pressure inside the can causing the can to become crushed under the pressure of the atmosphere. 4. Goal for Learner Students should be able to use Boyle’s law or Charles’ law successfully to solve for a change in either the pressure, volume or temperature of a gas. Students should be able to distinguish between Boyle’s and Charles’ law Students should be able to determine which law to utilize in order to solve any problems given on the worksheet or future assessments. 5. Content and Procedures Boyle’s Law o Robert Boyle used a J shaped tube which was closed on one side to determine the relationship between the pressure and volume of a gas. He filled the tube with a certain amount of mercury and noted the volume. He then added more mercury to the tube and noticed that the volume of the gas decreased as more mercury was added. He then multiplied the pressure and volume observations and noticed that the product of these values remained constant. This observation only holds true at a constant temperature and moles of gas. o If we remember that gas molecules must be in constant motion and constantly colliding with the container’s surface… we can visually see how the pressure of the gas must increase as the volume decreases. Because the volume of the gas decreases as the pressure increases, or The volume of the gas increases as the pressure decreases We say that the pressure and the volume of a gas are inversely proportional to one another. We can mathematically represent Boyle’s Law with the equation P1V1=P2V2 Boyle’s Law practice o Example A sample of neon to be used in a sign has a volume of 1.51 L under a pressure of 635 torr. Calculate the pressure of the neon gas when it is pumped into the tubes of the sign at a pressure of 785 torr. Revised 4/11 o Example If the pressure of 2.4 L of gas in a balloon is 1.35 atm, what is the pressure if the volume is increased to 5.4 L? Charles’ Law o Jacques Charles 1st person to fill a balloon with hydrogen gas 1st person to make a solo hot air balloon voyage Studied the relationship between volume and temperature Determined that the volume of the gas increases as the temperature of the gas is raised. o This means there is a direct correlation between the volume and temperature of a gas. o IMPORTANT- you need all temperatures, when dealing with gases, to be in Kelvin. o Absolute zero From the data collected by Charles, it can be extrapolated what the temperature would be of a gas when the volume is decreased to zero. We have to extrapolate because all gases liquefy before reaching this lowest possible temperature. This temperature is called Absolute Zero. This is the lowest possible temperature possible Is a theoretical value; has not been reached but have gotten close Is the temperature in which there is no molecular motion. Temperature is the average kinetic energy of the particles. o Volume and Temperature Charles found that the volume of a gas decreased when the temperature of the gas was decreased. He also noted that the volume would increase upon increasing the temperature. This is called a direct correlation. Both factors increase or decrease together. Charles’ Law is summarized in the equation Practice with Charles’ Law o Example If the volume of a gas at 25 °C is 13.6 L, what is the volume of the same gas at 35 °C? If the volume of a gas is 22.4 L at 25°C, what is the temperature when the volume is 12.2L? 6. Practice/Application Students are to complete the worksheet over Boyle’s law and Charles’ law to help students gain familiarity with the equations to be used throughout the chapter. The worksheet will be given to students after the lesson has been taught. Students are to work on the worksheet for the remainder of the class period, if time permits. 7. Evaluation of student learning The instructor should make use of both formal and inform evaluations of student performance to judge if any accommodations or alterations to future lesson plans need to be made. o Formal Revised 4/11 The worksheet that students complete at the completion of the lesson should be collected and graded for correctness. Based on student performance, any adjustments to future lessons, such as the addition of a review activity at the beginning of subsequent lessons may be deemed necessary. o Informal During the lecture portion of the lesson, the instructor should make sure to include many probing questions to assess that students are following along with the material and understanding the concepts being discussed throughout the lesson. 8. Closure Before letting students begin to work on the worksheet over Boyle’s and Charles’ Laws, the instructor should make sure to tell students how the demonstration at the beginning of the lesson was achieved. o The instructor should have students try to provide an explanation of the demonstration before giving the correct answer. The reason this happens is because as the temperature of the gas decreases the volume the gas occupies decreases, so the pressure from the atmosphere pushes on the balloon forcing it into the flask. Revised 4/11