Summer Packet
advertisement

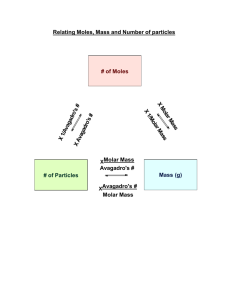
AP Chemistry Summer Packet Welcome to AP Chemistry! I’m so glad that you decided to take this course. This class will be a great challenge for you, but will do wonders in helping to prepare you for the rigors of college. AP Chemistry builds upon the concepts that were covered in first year chemistry, and it is my expectation that you remember a majority of that content. On day 1 of AP Chem, we will begin learning new material, and in talking with my AP Chem students from this past school year, they told me that they wished there had been a summer review so that they were prepared when they entered class the first day. Thus, I have created this packet. It is your first assignment for AP Chemistry and it is due at the beginning of the first day of class. With the exception of polyatomic ions, ALL of it is review from regular chemistry. I have posted the answer key to all of the worksheets on my website, http://www.usd305.com/Page/9019 as well as including additional websites, videos, and resources to help you should you need extra help or review of the content. I realize that you could easily go online and just copy my answers, but considering you have decided to take one of the most difficult classes offered to high school students, I know you are the kind of student who wants to be prepared for such a challenging course, and you will complete the work and use the answers on my website to check your understanding. Feel free to contact me if you have any questions via email (nikki.chamberlain@usd305.com) and I will help explain problems. Good luck and I’ll see you in a few months! Enjoy your summer break! Mrs. Chamberlain 1|Page Symbols We will take a symbols quiz on the first day of class. Do you remember these element symbols? It might be helpful to make flashcards or to try out these online games. http://education.jlab.org/elementflashcards/index.html http://quizhub.com/quiz/f-elements.cfm http://www.syvum.com/cgi/online/serve.cgi/squizzes/chem/periodic2.tdf 1. H 2. Li 3. Na 4. K 5. Rb 6. Cs 7. Fr 8. Be 9. Mg 10. Ca 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. Sr Ba Ra Sc Ti Zr V Cr Mo W 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. Mn Fe Ru Co Ni Pd Pt Cu Zn Cd 31. 32. 33. 34. 35. 36. 37. 38. 39. 40. Hg B Al Ga C Si Ge Sn Pb N 41. 42. 43. 44. 45. 46. 47. 48. 49. 50. 51. O S Se F Cl Br I At He Ne Sb 52. 53. 54. 55. 56. 57. 58. 59. 60. 61. 62. Ar Kr Xe Rn La Ac U Pu Ag Au P 2|Page Polyatomic Ions & Acids You will take a quiz over these polyatomic ions & acids the second day of class. You did not have to memorize polyatomic ions in first year chemistry (they were on the back of your Periodic Table), but in AP you will have to know them. Consider making flash cards. acetate acetic acid ammonium carbonate carbonic acid chlorate chloric acid chlorite chromate cyanide dichromate dihydrogen phosphate hydrobromic acid hydrogen carbonate (bicarbonate) hydrogen sulfate (bisulfate) hydrogen sulfide hydrogen sulfite (bisulfite) hydroiodic acid hydronium hydroxide hypochlorite iodate nitrate nitrite nitric acid nitrous acid oxalate perchlorate perchloric acid permaganate peroxide phosphate phosphite phosphoric acid silicate sulfate sulfite sulfuric acid thiocyanate 3|Page Significant Figures Review of the rules: 1. All nonzero numbers are significant (123 has 3 sig figs) 2. Zeros between nonzero numbers are significant (102 has 3 sig figs) 3. Zeros in front of nonzero numbers are not significant (0.0000123 has 3 sig figs) 4. Zeros at the end of a number and to the right of a decimal are significant (1.000 has 4 sig figs, 0.0002300 has 4 sig figs) 5. Zeros at the end of a number are not significant. If there is a decimal at the end, they are (100 has 1 sig fig, but 100. Has 3) 6. When multiplying and dividing, your answer has as many sig figs as the number in the problem with the fewest. 1. Indicate how many significant figures (sig figs) are in the following numbers: a) 4,000,001 b) 4,000,000 c) 4,000,000. d) 4.23 x 108 _____ _____ _____ _____ e) 0.000052 _____ f) 0.00005200 _____ g) 0 .000052001 _____ h) 1.280 x 10-5 _____ 2. Write the following numbers in scientific notation so that they each have 3 significant figures: a. 300 _______________________ b. 146,000 _______________________ c. 0.00120 _______________________ 3. Round the following numbers so that they contain 3 significant figures. a. 173,792 ______________________ b. 0.0025021 ______________________ c. 0.0003192 ______________________ 4. Solve the following. Use the appropriate sig figs in your answers: a. 0.000030 x 0.00002 x 0.06 = _______________________ b. 0.276 x 13.76 x 0.0100 = ________________________ c. 1,234 x 3 = ________________________ d. 3.8890/7 = _______________________ e. 1,000/3.9876 = _______________________ 4|Page Chemical Nomenclature Before you can name a compound or write a formula, you have to identify if it is ionic or covalent. Ionic is made of metals and nonmetal OR cations (+) and anions (-). Covalent compounds (molecular) are made of nonmetals only. Review of Rules: Type of Rules for Naming Compound Ionic 1. Name the metal (cation) If it is a multivalent element, use a roman numeral to indicate charge 2. Name the anion If the anion is a nonmetal use an “ide” ending If the anion is a polyatomic ion, just name it Covalent/ Molecular Rules for Writing Formula 1. Write each ion with its charge 2. Add subscripts to balance the charges (criss-cross the charges) 3. The overall charge on an ionic compound should be ZERO! 4. Simplify the formula if possible Examples: NaCl = sodium chloride NaNO3 = sodium nitrate Fe2(CO3)3 = Iron (III) carbonate 1. Name each element and use the appropriate prefix to tell how many Omit mono on the first element 2. Put an “ide” ending on the last element Examples: Calcium chloride = Ca+2Cl-1 = CaCl2 Sodium sulfate = Na+1SO4-2 = Na2SO4 Copper (II) oxide = Cu+2O-2 = CuO 1. Use the prefixes to determine the subscripts in the formula 2. Do NOT simplify molecular formulas Examples: P4O10 = tetraphosphorus decabromide CO = carbon monoxide Examples: Dinitrogen pentachloride = N2Cl5 Trihydrogen hexabromine octaoxide = H3Br6O8 Use the rules to write formulas and name the compounds on page 6. 5|Page Write names: 1. CO2 2. Co(CN)3 3. OF2 4. Zn(OH)2 5. NiCO3 6. CuSO4 7. KMnO4 8. (NH4)3PO4 9. C7F9 10.Mg(C2H3O2)2 Write formulas: 11. Copper (II) sulfate 12.Tricarbon nonachloride 13. Ammonium oxalate 14. Iron (II) oxide 15. Silver sulfate 16. Lead (IV) chromate 17.Calcium carbonate 18. Carbon monoxide 19. Cesium phosphide 20. Sodium hydroxide 6|Page Molar Conversions #grams 1 mole Mass in Molar Mass Grams PT 1 mole 6.022 X 1023 molecules Moles 6.022 X 1023 Molecules Or Atoms 1. Convert to moles: a. 32.9 g neon 1.63 moles b. 6.4 x 10-4 kg aluminum phosphate 5.3 x 10-3 moles c. 7.12 x 1023 molecules silicon tetrafluoride 1.18 moles 2. Convert to grams: a. 3.46 moles lithium carbonate 255 g 3. Convert to molecules: a. 6.72 moles sodium chloride 4.04 x 1024 molecules 7|Page b. 16.2 kg diphosphorus pentoxide 6.87 x 1025 molecules 4. Convert to grams: a. 1.06 x 1027 atoms copper 1.12 x 105 g b. 7.39 x 1023 molecules tin (II) chromate 288 g 5. The density of liquid water is 0.997 g/mL at 25 degrees Celsius. a. How many moles of water are in 250.0 mL of water? b. Calculate the volume that would be occupied by 2.00 moles of liquid water at 25 degrees Celsius? a. 13.85 mol H2O b. 36.1 mL H2O 8|Page Writing & Balancing Equations Review of Reaction Types: Type of Reaction Relationship Analogy Synthesis Dating Decomposition Break Up Single Cheating Replacement Double Swapping Replacement Combustion O2 Cute General Equation Example A + X AX AX A + X AX + B BX + A AX + Y AY + X AX + BY AY + BX N2 + 3H2 2NH3 CaCO3 CaO + CO2 Mg + 2HCl MgCl2 + H2 NaOH + AgCl NaCl + AgOH CxHy + O2 CO2 + H2O CH4 +2 O2 CO2 + 2H2O Remember that decomposition reactions are difficult to predict. For a review of them, visit this website http://quizlet.com/6084569/types-of-decomposition-reactions-flash-cards/ or access the link on my website. Remember to write water, in a reaction, as H+1OH-1 1. sodium iodide + chlorine 2. silver nitrate + potassium iodide 3. rubidium + bromine 4. sodium + water 5. magnesium chlorate 9|Page 6. silver nitrate + calcium 7. carbon tetrahydride + oxygen 8. bromine + calcium iodide 9. nitric acid + zinc 10. sulfuric acid + sodium chloride 11. silver carbonate 12. aluminum + sulfuric acid 10 | P a g e Stoichiometry 1. Sodium reacts with water to produce sodium hydroxide and hydrogen gas. a. How many moles of sodium will react if 4 moles of hydrogen are produced? 8 moles Na 2. In a very violent reaction called a thermite reaction, aluminum metal reacts with iron (III) oxide to form iron metal and aluminum oxide according to the following equation: Fe2O3 + 2Al 2Fe + Al2O3 a. If 0.905 mole Al2O3 is produced in the reaction, what mass of Fe is produced? 101 g Fe b. How many moles of Fe2O3 will react with 99.0 g of Al? 1.83 mole Fe2O3 11 | P a g e 3. Iron (III) chloride, FeCl3, can be made by the reaction of iron and chlorine gas. How much iron, in grams, will be needed to completely react with 58.0 g of Cl2? 30.4 g Fe 4. Calculate the mass of silver bromide produced from 22.5 g of silver nitrate in the following reaction: 2AgNO3 + MgBr2 2AgBr + Mg(NO3)2 24.9 g AgBr 5. Iron (III) hydroxide decomposes to produce iron (III) oxide and water vapor. If 0.75 L of water vapor is produced at STP, how many grams of iron (III) hydroxide were produced? 2.4 g Fe(OH)3 6. How many molecules of oxygen are produced by the decomposition of 6.54 g of potassium chlorate? 4.82 x 1022 molecules O2 12 | P a g e