Chapter 6 - Introductory & Human Biology
advertisement

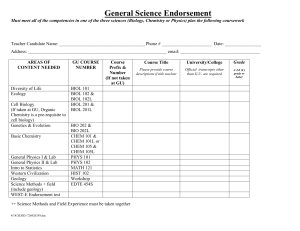
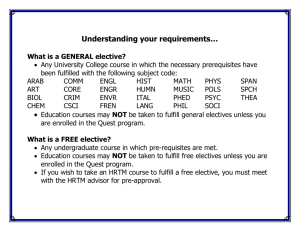
Chapter 6 6.1 Introduction Review Hille, B., 2001. Ion channels of excitable membranes. Sinauer Associates, Inc. Sunderland, MA. Hille, B., Armstrong, C. M., and MacKinnon, R., 1999. Ion channels: from idea to reality. Nat. Med. v. 5 p. 1105–1109. 6.2 Channels and carriers are the main types of membrane transport proteins Research Jentsch, T. J., Hübner, C. A., and Fuhrmann, J. C., 2004. Ion channels: Function unravelled by dysfunction. Nat. Cell Biol. v. 6 p. 1039–1047. + 6.5 K channels catalyze selective and rapid ion permeation Review Berneche, S., and Roux, B., 2001. Energetics of ion conduction through the K+ channel. Nature v. 414 p. 73–77. Choe, S., 2002. Potassium channel structures. Nat. Rev. Neurosci. v. 3 p. 115–121. Hille, B., Armstrong, C. M., and MacKinnon, R., 1999. Ion channels: from idea to reality. Nat. Med. v. 5 p. 1105–1109. Miller, C., 2000. An overview of the potassium channel family. Genome Biol. v. 1 p. R0004. Morais-Cabral, J. H., Zhou, Y., and MacKinnon, R., 2001. Energetic optimization of ion conduction rate by the K+ selectivity filter. Nature v. 414 p. 37–42. Research Doyle, D. A., Morais Cabral, J., Pfuetzner, R. A., Kuo, A., Gulbis, J. M., Cohen, S. L., Chait, B. T., and MacKinnon, R., 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science v. 280 p. 69–77. Gutman, G. A., et al., 2003. International Union of Pharmacology. XLI. Compendium of voltage-gated ion channels: potassium channels. Pharmacol. Rev. v. 55 p. 583– 586. Zhou, Y., Morais-Cabral, J. H., Kaufman, A., and MacKinnon, R., 2001. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 Å resolution. Nature v. 414 p. 43–48. 6.6 Different K+ channels use a similar gate coupled to different activating or inactivating mechanisms Review Choe, S., 2002. Potassium channel structures. Nat. Rev. Neurosci. v. 3 p. 115–121. Jiang, Y., Lee, A., Chen, J., Cadene, M., Chait, B. T., and MacKinnon, R., 2002. The open pore conformation of potassium channels. Nature v. 417 p. 523–526. Jiang, Y., Ruta, V., Chen, J., Lee, A., and MacKinnon, R., 2003. The principle of gating charge movement in a voltage-dependent K+ channel. Nature v. 423 p. 42–48. Kullmann, D. M., Rea, R., Spauschus, A., and Jouvenceau, A., 2001. The inherited episodic ataxias: how well do we understand the disease mechanisms? Neuroscientist v. 7 p. 80–88. Tristani-Firouzi, M., and Sanguinetti, M. C., 2003. Structural determinants and biophysical properties of HERG and KCNQ1 channel gating. J. Mol. Cell. Cardiol. v. 35 p. 27–35. Research Jiang, Y., Lee, A., Chen, J., Cadene, M., Chait, B. T., and MacKinnon, R., 2002. Crystal structure and mechanism of a calcium-gated potassium channel. Nature v. 417 p. 515–522. Long, S. B., Campbell, E. B., and MacKinnon, R., 2005. Voltage sensor of Kv1.2: structural basis of electromechanical coupling. Science v. 309 p. 903–908. Long, S. B., Campbell, E. B., and MacKinnon, R., 2005. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science v. 309 p. 897– 903. Zhou, Y., Morais-Cabral, J. H., Kaufman, A., and MacKinnon, R., 2001. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 Å resolution. Nature v. 414 p. 43–48. 6.7 Voltage-dependent Na+ channels are activated by membrane depolarization and translate electrical signals Review Hondeghem, L. M., and Katzung, B. G., 1977. Time- and voltage-dependent interactions of antiarrhythmic drugs with cardiac sodium channels. Biochim. Biophys. Acta v. 472 p. 373–398. Vilin, Y. Y., and Ruben, P. C., 2001. Slow inactivation in voltage-gated sodium channels: molecular substrates and contributions to channelopathies. Cell Biochem. Biophys. v. 35 p. 171–190. Yu, F. H., and Catterall, W. A., 2003. Overview of the voltage-gated sodium channel family. Genome Biol. v. 4 p. 207–207. Research Chiamvimonvat, N., Pérez-García, M. T., Ranjan, R., Marban, E., and Tomaselli, G. F., 1996. Depth asymmetries of the pore-lining segments of the Na+ channel revealed by cysteine mutagenesis. Neuron v. 16 p. 1037–1047. Isom, L. L., Ragsdale, D. S., De Jongh, K. S., Westenbroek, R. E., Reber, B. F., Scheuer, T., and Catterall, W. A., 1995. Structure and function of the beta 2 subunit of brain sodium channels, a transmembrane glycoprotein with a CAM motif. Cell v. 83 p. 433–442. Kellenberger, S., Scheuer, T., and Catterall, W. A., 1996. Movement of the Na+ channel inactivation gate during inactivation. J. Biol. Chem. v. 271 p. 30971–30979. Mitrovic, N., George, A. L., and Horn, R., 2000. Role of domain 4 in sodium channel slow inactivation. J. Gen. Physiol. v. 115 p. 707–718. Ragsdale, D. S., McPhee, J. C., Scheuer, T., and Catterall, W. A., 1996. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proc. Natl. Acad. Sci. USA v. 93 p. 9270– 9275. Rohl, C. A., Boeckman, F. A., Baker, C., Scheuer, T., Catterall, W. A., and Klevit, R. E., 1999. Solution structure of the sodium channel inactivation gate. Biochemistry v. 38 p. 855–861. Stühmer, W., Conti, F., Suzuki, H., Wang, X. D., Noda, M., Yahagi, N., Kubo, H., and Numa, S., 1989. Structural parts involved in activation and inactivation of the sodium channel. Nature v. 339 p. 597–603. 6.8 Epithelial Na+ channels regulate Na+ homeostasis Review Kellenberger, S., and Schild, L., 2002. Epithelial sodium channel/degenerin family of ion channels: a variety of functions for a shared structure. Physiol. Rev. v. 82 p. 735– 767. Research Bruns, J. B., Hu, B., Ahn, Y. J., Sheng, S., Hughey, R. P., and Kleyman, T. R., 2003. Multiple epithelial Na+ channel domains participate in subunit assembly. Am. J. Physiol. Renal Physiol. v. 285 p. F600–F609. Canessa, C. M., Schild, L., Buell, G., Thorens, B., Gautschi, I., Horisberger, J. D., and Rossier, B. C., 1994. Amiloride-sensitive epithelial Na+ channel is made of three homologous subunits. Nature v. 367 p. 463–467. Chang, S. S., et al., 1996. Mutations in subunits of the epithelial sodium channel cause salt wasting with hyperkalaemic acidosis, pseudohypoaldosteronism type 1. Nat. Genet. v. 12 p. 248–253. Hansson, J. H., Nelson-Williams, C., Suzuki, H., Schild, L., Shimkets, R., Lu, Y., Canessa, C., Iwasaki, T., Rossier, B., and Lifton, R. P., 1995. Hypertension caused by a truncated epithelial sodium channel gamma subunit: genetic heterogeneity of Liddle syndrome. Nat. Genet. v. 11 p. 76–82. Palmer, L. G., and Andersen, O. S., 1989. Interactions of amiloride and small monovalent cations with the epithelial sodium channel. Inferences about the nature of the channel pore. Biophys. J. v. 55 p. 779–787. Reif, M. C., Troutman, S. L., and Schafer, J. A., 1986. Sodium transport by rat cortical collecting tubule. Effects of vasopressin and desoxycorticosterone. J. Clin. Invest. v. 77 p. 1291–1298. 6.9 Plasma membrane Ca2+ channels activate intracellular functions Review Carafoli, E., 2003. The calcium-signalling saga: tap water and protein crystals. Nat. Rev. Mol. Cell Biol. v. 4 p. 326–332. Sather, W. A., and McCleskey, E. W., 2003. Permeation and selectivity in calcium channels. Annu. Rev. Physiol. v. 65 p. 133–159. Research Ellinor, P. T., Yang, J., Sather, W. A., Zhang, J. F., and Tsien, R. W., 1995. Ca2+ channel selectivity at a single locus for high-affinity Ca2+ interactions. Neuron v. 15 p. 1121–1132. Erickson, M. G., Liang, H., Mori, M. X., and Yue, D. T., 2003. FRET two-hybrid mapping reveals function and location of L-type Ca2+ channel CaM preassociation. Neuron v. 39 p. 97–107. Hess, P., and Tsien, R. W., 1984. Mechanism of ion permeation through calcium channels. Nature v. 309 p. 453–456. Liang, H., DeMaria, C. D., Erickson, M. G., Mori, M. X., Alseikhan, B. A., and Yue, D. T., 2003. Unified mechanisms of Ca2+ regulation across the Ca2+ channel family. Neuron v. 39 p. 951–960. Lipkind, G. M., and Fozzard, H. A., 2003. Molecular modeling of interactions of dihydropyridines and phenylalkylamines with the inner pore of the L-type Ca2+ channel. Mol. Pharmacol. v. 63 p. 499–511. Serysheva, I. I., Ludtke, S. J., Baker, M. R., Chiu, W., and Hamilton, S. L., 2002. Structure of the voltage-gated L-type Ca2+ channel by electron cryomicroscopy. Proc. Natl. Acad. Sci. USA v. 99 p. 10370–10375. Wu, X. S., Edwards, H. D., and Sather, W. A., 2000. Side chain orientation in the selectivity filter of a voltage-gated Ca2+ channel. J. Biol. Chem. v. 275 p. 31778– 31785. Yang, J., Ellinor, P. T., Sather, W. A., Zhang, J. F., and Tsien, R. W., 1993. Molecular determinants of Ca2+ selectivity and ion permeation in L-type Ca2+ channels. Nature v. 366 p. 158–161. – 6.10 Cl channels serve diverse biological functions Review Bretag, A. H., 1987. Muscle chloride channels. Physiol. Rev. v. 67 p. 618–724. Ellison, D. H., 2000. Divalent cation transport by the distal nephron: insights from Bartter’s and Gitelman’s syndromes. Am. J. Physiol. Renal Physiol. v. 279 p. F616–F625. Jentsch, T. J., Stein, V., Weinreich, F., and Zdebik, A. A., 2002. Molecular structure and physiological function of chloride channels. Physiol. Rev. v. 82 p. 503–568. Research Accardi, A., and Miller, C., 2004. Secondary active transport mediated by a prokaryotic homologue of ClC Cl- channels. Nature v. 427 p. 803–807. Birkenhäger, R., et al., 2001. Mutation of BSND causes Bartter syndrome with sensorineural deafness and kidney failure. Nat. Genet. v. 29 p. 310–314. Dutzler, R., Campbell, E. B., and MacKinnon, R., 2003. Gating the selectivity filter in ClC chloride channels. Science v. 300 p. 108–112. Dutzler, R., Campbell, E. B., Cadene, M., Chait, B. T., and MacKinnon, R., 2002. X-ray structure of a ClC chloride channel at 3.0 Å reveals the molecular basis of anion selectivity. Nature v. 415 p. 287–294. Koch, M. C., Steinmeyer, K., Lorenz, C., Ricker, K., Wolf, F., Otto, M., Zoll, B., Lehmann-Horn, F., Grzeschik, K. H., and Jentsch, T. J., 1992. The skeletal muscle chloride channel in dominant and recessive human myotonia. Science v. 257 p. 797–800. Kornak, U., Kasper, D., Bösl, M. R., Kaiser, E., Schweizer, M., Schulz, A., Friedrich, W., Delling, G., and Jentsch, T. J., 2001. Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell v. 104 p. 205–215. Lloyd, S. E., et al., 1996. A common molecular basis for three inherited kidney stone diseases. Nature v. 379 p. 445–449. Miller, C., and White, M. M., 1984. Dimeric structure of single chloride channels from Torpedo electroplax. Proc. Natl. Acad. Sci. USA v. 81 p. 2772–2775. Pusch, M., Ludewig, U., Rehfeldt, A., and Jentsch, T. J., 1995. Gating of the voltagedependent chloride channel CIC-0 by the permeant anion. Nature v. 373 p. 527– 531. Simon, D. B., et al., 1997. Mutations in the chloride channel gene, CLCNKB, cause Bartter’s syndrome type III. Nat. Genet. v. 17 p. 171–178. 6.11 Selective water transport occurs through aquaporin channels Review Lehmann, G. L., Gradilone, S. A., and Marinelli, R. A., 2004. Aquaporin water channels in central nervous system. Curr. Neurovasc. Res. v. 1 p. 293–303. Nielsen, S., Frøkiaer, J., Marples, D., Kwon, T. H., Agre, P., and Knepper, M. A., 2002. Aquaporins in the kidney: from molecules to medicine. Physiol. Rev. v. 82 p. 205–244. Valenti, G., Procino, G., Tamma, G., Carmosino, M., and Svelto, M., 2005. Minireview: aquaporin 2 trafficking. Endocrinology v. 146 p. 5063–5070. Verkman, A. S., 2005. More than just water channels: unexpected cellular roles of aquaporins. J. Cell Sci. v. 118 p. 3225–3232. Research Deen, P. M., Verdijk, M. A., Knoers, N. V., Wieringa, B., Monnens, L. A., van Os, C. H., and van Oost, B. A., 1994. Requirement of human renal water channel aquaporin2 for vasopressin-dependent concentration of urine. Science v. 264 p. 92–95. Harries, W. E., Akhavan, D., Miercke, L. J., Khademi, S., and Stroud, R. M., 2004. The channel architecture of aquaporin-0 at a 2.2-Å resolution. Proc. Natl. Acad. Sci. USA v. 101 p. 14045–14050. Jung, J. S., Preston, G. M., Smith, B. L., Guggino, W. B., and Agre, P., 1994. Molecular structure of the water channel through aquaporin CHIP. The hourglass model. J. Biol. Chem. v. 269 p. 14648–14654. King, L. S., Choi, M., Fernandez, P. C., Cartron, J. P., and Agre, P., 2001. Defective urinary-concentrating ability due to a complete deficiency of aquaporin-1. N. Engl. J. Med. v. 345 p. 175–179. Murata, K., Mitsuoka, K., Hirai, T., Walz, T., Agre, P., Heymann, J. B., Engel, A., and Fujiyoshi, Y., 2000. Structural determinants of water permeation through aquaporin-1. Nature v. 407 p. 599–605. Smith, B. L., and Agre, P., 1991. Erythrocyte Mr 28,000 transmembrane protein exists as a multisubunit oligomer similar to channel proteins. J. Biol. Chem. v. 266 p. 6407–6415. Sui, H., Han, B. G., Lee, J. K., Walian, P., and Jap, B. K., 2001. Structural basis of water-specific transport through the AQP1 water channel. Nature v. 414 p. 872– 878. Zeidel, M. L., Ambudkar, S. V., Smith, B. L., and Agre, P., 1992. Reconstitution of functional water channels in liposomes containing purified red cell CHIP28 protein. Biochemistry v. 31 p. 7436–7440. 6.12 Action potentials are electrical signals that depend on several types of ion channels Review Carmeliet, E., 2004. Intracellular Ca(2+) concentration and rate adaptation of the cardiac action potential. Cell Calcium v. 35 p. 557–573. Keating, M. T., and Sanguinetti, M. C., 2001. Molecular and cellular mechanisms of cardiac arrhythmias. Cell v. 104 p. 569–580. Nichols, C. G., and Lopatin, A. N., 1997. Inward rectifier potassium channels. Annu. Rev. Physiol. v. 59 p. 171–191. Sah, R., Ramirez, R. J., Oudit, G. Y., Gidrewicz, D., Trivieri, M. G., Zobel, C., and Backx, P. H., 2003. Regulation of cardiac excitation-contraction coupling by action potential repolarization: role of the transient outward potassium current (Ito). J. Physiol. v. 546 p. 5–18. Research Bennett, P. B., Yazawa, K., Makita, N., and George, A. L., 1995. Molecular mechanism for an inherited cardiac arrhythmia. Nature v. 376 p. 683–685. Chen, Q., et al., 1998. Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature v. 392 p. 293–296. Curran, M. E., Splawski, I., Timothy, K. W., Vincent, G. M., Green, E. D., and Keating, M. T., 1995. A molecular basis for cardiac arrhythmia: HERG mutations cause long QT syndrome. Cell v. 80 p. 795–803. Hodgkin, A. L., and Huxley, A. F., 1952. A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. v. 117 p. 500–544. Hodgkin, A. L. and Huxley, A. F., 1952. Propagation of electrical signals along giant nerve fibers. Proc. R Soc. Lond. B Biol. Sci. v. 140 p. 177–183. Hodgkin, A. L. and Huxley, A. F., 1952. Movement of sodium and potassium ions during nervous activity. Cold Spring Harb. Symp. Quant. Biol. v. 17 p. 43–52. Lossin, C., Wang, D. W., Rhodes, T. H., Vanoye, C. G., and George, A. L., 2002. Molecular basis of an inherited epilepsy. Neuron v. 34 p. 877–884. Schott, J. J., Alshinawi, C., Kyndt, F., Probst, V., Hoorntje, T. M., Hulsbeek, M., Wilde, A. A., Escande, D., Mannens, M. M., and Le Marec, H., 1999. Cardiac conduction defects associate with mutations in SCN5A. Nat. Genet. v. 23 p. 20– 21. Splawski, I., et al., 2004. Ca(V)1.2 calcium channel dysfunction causes a multisystem disorder including arrhythmia and autism. Cell v. 119 p. 19–31. Wang, Q., Shen, J., Splawski, I., Atkinson, D., Li, Z., Robinson, J. L., Moss, A. J., Towbin, J. A., and Keating, M. T., 1995. SCN5A mutations associated with an inherited cardiac arrhythmia, long QT syndrome. Cell v. 80 p. 805–811. 6.13 Cardiac and skeletal muscles are activated by excitation-contraction coupling Review Berchtold, M. W., Brinkmeier, H., and Müntener, M., 2000. Calcium ion in skeletal muscle: its crucial role for muscle function, plasticity, and disease. Physiol. Rev. v. 80 p. 1215–1265. Berridge, M. J., Bootman, M. D., and Roderick, H. L., 2003. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. v. 4 p. 517– 529. Bers, D. M., 2002. Cardiac excitation-contraction coupling. Nature v. 415 p. 198–205. da Fonseca, P. C., Morris, S. A., Nerou, E. P., Taylor, C. W., and Morris, E. P., 2003. Domain organization of the type 1 inositol 1,4,5-trisphosphate receptor as revealed by single-particle analysis Proc. Natl. Acad. Sci. USA v. 100 p. 3936– 3941. Rizzuto, R., and Pozzan, T., 2006. Microdomains of intracellular Ca2+: molecular determinants and functional consequences. Physiol. Rev. v. 86 p. 369–408. Wehrens, X. H., Lehnart, S. E., and Marks, A. R., 2005. Intracellular calcium release and cardiac disease. Annu. Rev. Physiol. v. 67 p. 69–98. Research Campbell, K. P., Knudson, C. M., Imagawa, T., Leung, A. T., Sutko, J. L., Kahl, S. D., Raab, C. R., and Madson, L., 1987. Identification and characterization of the high affinity [3H]ryanodine receptor of the junctional sarcoplasmic reticulum Ca2+ release channel. J. Biol. Chem. v. 262 p. 6460–6463. Lehnart, S. E., Wehrens, X. H., Reiken, S., Warrier, S., Belevych, A. E., Harvey, R. D., Richter, W., Jin, S. L., Conti, M., and Marks, A. R., 2005. Phosphodiesterase 4D deficiency in the ryanodine-receptor complex promotes heart failure and arrhythmias. Cell v. 123 p. 25–35. Marx, S. O., Reiken, S., Hisamatsu, Y., Jayaraman, T., Burkhoff, D., Rosemblit, N., and Marks, R., 2000. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell v. 101 p. 365–376. Sharma, M. R., Penczek, P., Grassucci, R., Xin, H. B., Fleischer, S., and Wagenknecht, T., 1998. Cryoelectron microscopy and image analysis of the cardiac ryanodine receptor. J. Biol. Chem. v. 273 p. 18429–18434. Tanabe, T., Beam, K. G., Adams, B. A., Niidome, T., and Numa, S., 1990. Regions of the skeletal muscle dihydropyridine receptor critical for excitation-contraction coupling. Nature v. 346 p. 567–569. Wehrens, X. H., Lehnart, S. E., Reiken, S. R., Deng, S. X., Vest, J. A., Cervantes, D., Coromilas, J., Landry, D. W., and Marks, A. R., 2004. Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science v. 304 p. 292–296. Wehrens, X. H. et al., 2003. FKBP12.6 deficiency and defective calcium release channel (ryanodine receptor) function linked to exercise-induced sudden cardiac death. Cell v. 113 p. 829–840. 6.14 Some glucose transporters are uniporters Review Devaskar, S. U., and Mueckler, M. M., 1992. The mammalian glucose transporters. Pediatr. Res. v. 31 p. 1–13. Kahn, B. B., 1992. Facilitative glucose transporters: regulatory mechanisms and dysregulation in diabetes. J. Clin. Invest. v. 89 p. 1367–1374. Saier, M. H., 2000. Families of transmembrane sugar transport proteins. Mol. Microbiol. v. 35 p. 699–710. Vannucci, S. J., Maher, F., and Simpson, I. A., 1997. Glucose transporter proteins in brain: delivery of glucose to neurons and glia. Glia v. 21 p. 2–21. Research Heilig, C. W., Saunders, T., Brosius, F. C., Moley, K., Heilig, K., Baggs, R., Guo, L., and Conner, D., 2003. Glucose transporter-1-deficient mice exhibit impaired development and deformities that are similar to diabetic embryopathy. Proc. Natl. Acad. Sci. USA v. 100 p. 15613–15618. Mueckler, M., Caruso, C., Baldwin, S. A., Panico, M., Blench, I., Morris, H. R., Allard, W. J., Lienhard, G. E., and Lodish, H. F., 1985. Sequence and structure of a human glucose transporter. Science v. 229 p. 941–945. Mueckler, M., and Makepeace, C., 2004. Analysis of transmembrane segment 8 of the GLUT1 glucose transporter by cysteine-scanning mutagenesis and substituted cysteine accessibility. J. Biol. Chem. v. 279 p. 10494–10499. Seidner, G., Alvarez, M. G., Yeh, J. I., O’Driscoll, K. R., Klepper, J., Stump, T. S., Wang, D., Spinner, N. B., Birnbaum, M. J., and De Vivo, D. C., 1998. GLUT-1 deficiency syndrome caused by haploinsufficiency of the blood-brain barrier hexose carrier. Nat. Genet. v. 18 p. 188–191. Shigematsu, S., Watson, R. T., Khan, A. H., and Pessin, J. E., 2003. The adipocyte plasma membrane caveolin functional/structural organization is necessary for the efficient endocytosis of GLUT4. J. Biol. Chem. v. 278 p. 10683–10690. 6.15 Symporters and antiporters mediate coupled transport Review Abramson, J., Smirnova, I., Kasho, V., Verner, G., Iwata, S., and Kaback, H. R., 2003. The lactose permease of Escherichia coli: overall structure, the sugar-binding site and the alternating access model for transport. FEBS Lett. v. 555 p. 96–101. Kaback, H. R., Sahin-Tóth, M., and Weinglass, A. B., 2001. The kamikaze approach to membrane transport. Nat. Rev. Mol. Cell Biol. v. 2 p. 610–620. Research Abramson, J., Smirnova, I., Kasho, V., Verner, G., Kaback, H. R., and Iwata, S., 2003. Structure and mechanism of the lactose permease of Escherichia coli. Science v. 301 p. 610–615. Huang, Y., Lemieux, M. J., Song, J., Auer, M., and Wang, D. N., 2003. Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science v. 301 p. 616–620. Jardetzky, O., 1966. Simple allosteric model for membrane pumps. Nature v. 211 p. 969– 970. 6.16 The transmembrane Na+ gradient is essential for the function of many transporters Review Blaustein, M. P., and Lederer, W. J., 1999. Sodium/calcium exchange: its physiological implications. Physiol. Rev. v. 79 p. 763–854. Ellison, D. H., 2000. Divalent cation transport by the distal nephron: insights from Bartter’s and Gitelman’s syndromes. Am. J. Physiol. Renal Physiol. v. 279 p. F616–F625. Kaplan, M. R., Mount, D. B., and Delpire, E., 1996. Molecular mechanisms of NaCl cotransport. Annu. Rev. Physiol. v. 58 p. 649–668. Rasgado-Flores, H., and Gonzalez-Serratos, H., 2000. Plasmalemmal transport of magnesium in excitable cells. Front. Biosci. v. 5 p. D866–D879. Webel, R., Haug-Collet, K., Pearson, B., Szerencsei, R. T., Winkfein, R. J., Schnetkamp, P. P., and Colley, N. J., 2002. Potassium-dependent sodium-calcium exchange through the eye of the fly. Ann. N Y Acad. Sci. v. 976 p. 300–314. Research Haug-Collet, K., Pearson, B., Webel, R., Szerencsei, R. T., Winkfein, R. J., Schnetkamp, P. P., and Colley, N. J., 1999. Cloning and characterization of a potassiumdependent sodium/calcium exchanger in Drosophila. J. Cell Biol. v. 147 p. 659– 670. Nicoll, D. A., Ottolia, M., Lu, L., Lu, Y., and Philipson, K. D., 1999. A new topological model of the cardiac sarcolemmal Na+-Ca2+ exchanger. J. Biol. Chem. v. 274 p. 910–917. Simon, D. B., Karet, F. E., Hamdan, J. M., DiPietro, A., Sanjad, S. A., and Lifton, R. P., 1996. Bartter’s syndrome, hypokalaemic alkalosis with hypercalciuria, is caused by mutations in the Na-K-2Cl cotransporter NKCC2. Nat. Genet. v. 13 p. 183– 188. Simon, D. B., Karet, F. E., Rodriguez-Soriano, J., Hamdan, J. H., DiPietro, A., Trachtman, H., Sanjad, S. A., and Lifton, R. P., 1996. Genetic heterogeneity of Bartter’s syndrome revealed by mutations in the K+ channel, ROMK. Nat. Genet. v. 14 p. 152–156. Simon, D. B., et al., 1997. Mutations in the chloride channel gene, CLCNKB, cause Bartter’s syndrome type III. Nat. Genet. v. 17 p. 171–178. Simon, D. B., et al., 1996. Gitelman’s variant of Bartter’s syndrome, inherited hypokalaemic alkalosis, is caused by mutations in the thiazide-sensitive Na-Cl cotransporter. Nat. Genet. v. 12 p. 24–30. 6.17 Some Na+ transporters regulate cytosolic or extracellular pH Review Bobulescu, I. A., Di Sole, F., and Moe, O. W., 2005. Na+/H+ exchangers: physiology and link to hypertension and organ ischemia. Curr. Opin. Nephrol. Hypertens. v. 14 p. 485–494. Fliegel, L., and Karmazyn, M., 2004. The cardiac Na-H exchanger: a key downstream mediator for the cellular hypertrophic effects of paracrine, autocrine and hormonal factors. Biochem. Cell Biol. v. 82 p. 626–635. Gross, E., and Kurtz, I., 2002. Structural determinants and significance of regulation of electrogenic Na+-HCO3– cotransporter stoichiometry. Am. J. Physiol. Renal Physiol. v. 283 p. F876–F887. Wakabayashi, S., Shigekawa, M., and Pouyssegur, J., 1997. Molecular physiology of vertebrate Na+/H+ exchangers. Physiol. Rev. v. 77 p. 51–74. Zachos, N. C., Tse, M., and Donowitz, M., 2005. Molecular physiology of intestinal Na+/H+ exchange. Annu. Rev. Physiol. v. 67 p. 411–443. Research Akiba, Y., Furukawa, O., Guth, P. H., Engel, E., Nastaskin, I., Sassani, P., Dukkipatis, R., Pushkin, A., Kurtz, I., and Kaunitz, J. D., 2001. Cellular bicarbonate protects rat duodenal mucosa from acid-induced injury. J. Clin. Invest. v. 108 p. 1807–1816. Hunte, C., Screpanti, E., Venturi, M., Rimon, A., Padan, E., and Michel, H., 2005. Structure of a Na+/H+ antiporter and insights into mechanism of action and regulation by pH. Nature v. 435 p. 1197–1202. Orlowski, J. and Grinstein, S., 2004. Diversity of the mammalian sodium/proton exchanger SLC9 gene family. Pflugers Arch. v. 447 p. 549–565. Williams, K. A., 2000. Three-dimensional structure of the ion-coupled transport protein NhaA. Nature v. 403 p. 112–115. 6.18 The Ca2+-ATPase pumps Ca2+ into intracellular storage compartments Review Belke, D. D., and Dillmann, W. H., 2004. Altered cardiac calcium handling in diabetes. Curr. Hypertens. Rep. v. 6 p. 424–429. Green, N. M., and MacLennan, D. H., 2002. Calcium callisthenics. Nature v. 418 p. 598– 599. Kühlbrandt, W., 2004. Biology, structure and mechanism of P-type ATPases. Nat. Rev. Mol. Cell Biol. v. 5 p. 282–295. Laporte, R., Hui, A., and Laher, I., 2004. Pharmacological modulation of sarcoplasmic reticulum function in smooth muscle. Pharmacol. Rev. v. 56 p. 439–513. Stokes, D. L., and Green, N. M., 2003. Structure and function of the calcium pump. Annu. Rev. Biophys. Biomol. Struct. v. 32 p. 445–468. Strehler, E. E., and Treiman, M., 2004. Calcium pumps of plasma membrane and cell interior. Curr. Mol. Med. v. 4 p. 323–335. Sweadner, K. J., and Donnet, C., 2001. Structural similarities of Na,K-ATPase and SERCA, the Ca2+-ATPase of the sarcoplasmic reticulum. Biochem. J. v. 356 p. 685–704. Verkhratsky, A., 2004. Endoplasmic reticulum calcium signaling in nerve cells. Biol. Res. v. 37 p. 693–699. Research Sørensen, T. L., Møller, J. V., and Nissen, P., 2004. Phosphoryl transfer and calcium ion occlusion in the calcium pump. Science v. 304 p. 1672–1675. Toyoshima, C., Nakasako, M., Nomura, H., and Ogawa, H., 2000. Crystal structure of the calcium pump of sarcoplasmic reticulum at 2.6 Å resolution. Nature v. 405 p. 647–655. Toyoshima, C., and Nomura, H., 2002. Structural changes in the calcium pump accompanying the dissociation of calcium. Nature v. 418 p. 605–611. 6.19 The Na+/K+-ATPase maintains the plasma membrane Na+ and K+ gradients Review Glitsch, H. G., 2001. Electrophysiology of the sodium-potassium-ATPase in cardiac cells. Physiol. Rev. v. 81 p. 1791–1826. Horisberger, J. D., 2004. Recent insights into the structure and mechanism of the sodium pump. Physiology (Bethesda) v. 19 p. 377–387. Kühlbrandt, W., 2004. Biology, structure and mechanism of P-type ATPases. Nat. Rev. Mol. Cell Biol. v. 5 p. 282–295. Rakowski, R. F., and Sagar, S., 2003. Found: Na+ and K+ binding sites of the sodium pump. News Physiol. Sci. v. 18 p. 164–168. Sweadner, K. J., and Donnet, C., 2001. Structural similarities of Na,K-ATPase and SERCA, the Ca2+-ATPase of the sarcoplasmic reticulum. Biochem. J. v. 356 p. 685–704. Research Hilge, M., Siegal, G., Vuister, G. W., Güntert, P., Gloor, S. M., and Abrahams, J. P., 2003. ATP-induced conformational changes of the nucleotide-binding domain of Na,K-ATPase. Nat. Struct. Biol. v. 10 p. 468–474. Rice, W. J., Young, H. S., Martin, D. W., Sachs, J. R., and Stokes, D. L., 2001. Structure of Na+,K+-ATPase at 11-Å resolution: comparison with Ca2+-ATPase in E1 and E2 states. Biophys. J. v. 80 p. 2187–2197. Toyoshima, C., and Nomura, H., 2002. Structural changes in the calcium pump accompanying the dissociation of calcium. Nature v. 418 p. 605–611. 6.20 The F1Fo-ATP synthase couples H+ movement to ATP synthesis or hydrolysis Review Senior, A. E., Nadanaciva, S., and Weber, J., 2002. The molecular mechanism of ATP synthesis by F1Fo-ATP synthase. Biochim. Biophys. Acta v. 1553 p. 188–211. Research Bernal, R. A. and Stock, D., 2004. Three-dimensional structure of the intact Thermus thermophilus H+-ATPase/synthase by electron microscopy. Structure (Camb) v. 12 p. 1789–1798. Itoh, H., Takahashi, A., Adachi, K., Noji, H., Yasuda, R., Yoshida, M., and Kinosita, K., 2004. Mechanically driven ATP synthesis by F1-ATPase. Nature v. 427 p. 465– 468. Senior, A. E. and Weber, J., 2004. Happy motoring with ATP synthase. Nat. Struct. Mol. Biol. v. 11 p. 110–112. Stock, D., Leslie, A. G., and Walker, J. E., 1999. Molecular architecture of the rotary motor in ATP synthase. Science v. 286 p. 1700–1705. 6.21 H+-ATPases transport protons out of the cytosol Review Bajjalieh, S., 2005. A new view of an old pore. Cell v. 121 p. 496–497. Brown, D., and Breton, S., 2000. H(+)V-ATPase-dependent luminal acidification in the kidney collecting duct and the epididymis/vas deferens: vesicle recycling and transcytotic pathways. J. Exp. Biol. v. 203 p. 137–145. Fillingame, R. H., Jiang, W., and Dmitriev, O. Y., 2000. Coupling H(+) transport to rotary catalysis in F-type ATP synthases: structure and organization of the transmembrane rotary motor. J. Exp. Biol. v. 203 Pt 1 p. 9–17. Grüber, G., Wieczorek, H., Harvey, W. R., and Müller, V., 2001. Structure-function relationships of A-, F- and V-ATPases. J. Exp. Biol. v. 204 p. 2597–2605. Karet, F. E., 2002. Monogenic tubular salt and acid transporter disorders. J. Nephrol. v. 15 Suppl 6 p. S57–S68. Nishi, T., and Forgac, M., 2002. The vacuolar (H+)-ATPases—nature’s most versatile proton pumps. Nat. Rev. Mol. Cell Biol. v. 3 p. 94–103. Stevens, T. H., and Forgac, M., 1997. Structure, function and regulation of the vacuolar (H+)-ATPase. Annu. Rev. Cell Dev. Biol. v. 13 p. 779–808. Vik, S. B., Long, J. C., Wada, T., and Zhang, D., 2000. A model for the structure of subunit a of the Escherichia coli ATP synthase and its role in proton translocation. Biochim. Biophys. Acta v. 1458 p. 457–466. Research Arai, H., Terres, G., Pink, S., and Forgac, M., 1988. Topography and subunit stoichiometry of the coated vesicle proton pump. J. Biol. Chem. v. 263 p. 8796– 8802. Kane, P. M., 1995. Disassembly and reassembly of the yeast vacuolar H+-ATPase in vivo. J. Biol. Chem. v. 270 p. 17025–17032. Smith, A. N., Lovering, R. C., Futai, M., Takeda, J., Brown, D., and Karet, F. E., 2003. Revised nomenclature for mammalian vacuolar-type H+-ATPase subunit genes. Mol. Cell v. 12 p. 801–803. Vasilyeva, E., Liu, Q., MacLeod, K. J., Baleja, J. D., and Forgac, M., 2000. Cysteine scanning mutagenesis of the noncatalytic nucleotide binding site of the yeast VATPase. J. Biol. Chem. v. 275 p. 255–260. Wilkens, S., Vasilyeva, E., and Forgac, M., 1999. Structure of the vacuolar ATPase by electron microscopy. J. Biol. Chem. v. 274 p. 31804–31810. 6.22 What’s next? Review Multiple authors, 2004. The state of ion channel research in 2004. Nat. Rev. Drug Discov. v. 3 p. 239–278. 6.25 Supplement: Most K+ channels undergo rectification Review Ashcroft, F. M., 1988. Adenosine 5’-triphosphate-sensitive potassium channels. Annu. Rev. Neurosci. v. 11 p. 97–118. Ashcroft, F. M., and Gribble, F. M., 1999. ATP-sensitive K+ channels and insulin secretion: their role in health and disease. Diabetologia v. 42 p. 903–919. Bichet, D., Haass, F. A., and Jan, L. Y., 2003. Merging functional studies with structures of inward-rectifier K+ channels. Nat. Rev. Neurosci. v. 4 p. 957–967. Campbell, J. D., Sansom, M. S., and Ashcroft, F. M., 2003. Potassium channel regulation. EMBO Rep. v. 4 p. 1038–1042. Dhamoon, A. S., and Jalife, J., 2005. The inward rectifier current (IK1) controls cardiac excitability and is involved in arrhythmogenesis. Heart Rhythm v. 2 p. 316–324. Lu, Z., 2004. Mechanism of rectification in inward-rectifier K+ channels. Annu. Rev. Physiol. v. 66 p. 103–129. Nichols, C. G., and Lopatin, A. N., 1997. Inward rectifier potassium channels. Annu. Rev. Physiol. v. 59 p. 171–191. Research Kuo, A., Gulbis, J. M., Antcliff, J. F., Rahman, T., Lowe, E. D., Zimmer, J., Cuthbertson, J., Ashcroft, F. M., Ezaki, T., and Doyle, D. A., 2003. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science v. 300 p. 1922–1926. Liu, Y., Jurman, M. E., and Yellen, G., 1996. Dynamic rearrangement of the outer mouth of a K+ channel during gating. Neuron v. 16 p. 859–867. Reimann, F., Huopio, H., Dabrowski, M., Proks, P., Gribble, F. M., Laakso, M., Otonkoski, T., and Ashcroft, F. M., 2003. Characterisation of new KATP-channel mutations associated with congenital hyperinsulinism in the Finnish population. Diabetologia v. 46 p. 241–249. 6.26 Supplement: Mutations in an anion channel cause cystic fibrosis Review Higgins, C. F., 2001. ABC transporters: physiology, structure and mechanism—an overview. Res. Microbiol. v. 152 p. 205–210. Riordan, J. R., 2005. Assembly of functional CFTR chloride channels. Annu. Rev. Physiol. v. 67 p. 701–718. Sheppard, D. N., and Welsh, M. J., 1999. Structure and function of the CFTR chloride channel. Physiol. Rev. v. 79 p. S23–S45. Slieker, M. G., Sanders, E. A., Rijkers, G. T., Ruven, H. J., and van der Ent, C. K., 2005. Disease modifying genes in cystic fibrosis. J. Cyst. Fibros. v. 4 Suppl 2 p. 7–13. Steward, M. C., Ishiguro, H., and Case, R. M., 2005. Mechanisms of bicarbonate secretion in the pancreatic duct. Annu. Rev. Physiol. v. 67 p. 377–409. Research Bear, C. E., Li, C. H., Kartner, N., Bridges, R. J., Jensen, T. J., Ramjeesingh, M., and Riordan, J. R., 1992. Purification and functional reconstitution of the cystic fibrosis transmembrane conductance regulator (CFTR). Cell v. 68 p. 809–818. Bishop, L., Agbayani, R., Ambudkar, S. V., Maloney, P. C., and Ames, G. F., 1989. Reconstitution of a bacterial periplasmic permease in proteoliposomes and demonstration of ATP hydrolysis concomitant with transport. Proc. Natl. Acad. Sci. USA v. 86 p. 6953–6957. Chang, G., and Roth, C. B., 2001. Structure of MsbA from E. coli: a homolog of the multidrug resistance ATP binding cassette (ABC) transporters. Science v. 293 p. 1793–1800. Knowles, M. R., Stutts, M. J., Spock, A., Fischer, N., Gatzy, J. T., and Boucher, R. C., 1983. Abnormal ion permeation through cystic fibrosis respiratory epithelium. Science v. 221 p. 1067–1070. Mall, M., Grubb, B. R., Harkema, J. R., O’Neal, W. K., and Boucher, R. C., 2004. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat. Med. v. 10 p. 487–493. Quinton, P. M., 1983. Chloride impermeability in cystic fibrosis. Nature v. 301 p. 421– 422. Randak, C., and Welsh, M. J., 2003. An intrinsic adenylate kinase activity regulates gating of the ABC transporter CFTR. Cell v. 115 p. 837–850. Reddy, M. M., Light, M. J., and Quinton, P. M., 1999. Activation of the epithelial Na+ channel (ENaC) requires CFTR Cl- channel function. Nature v. 402 p. 301–304. Reddy, M. M., and Quinton, P. M., 2003. Control of dynamic CFTR selectivity by glutamate and ATP in epithelial cells. Nature v. 423 p. 756–760. Riordan, J. R., Rommens, J. M., Kerem, B., Alon, N., Rozmahel, R., Grzelczak, Z., Zielenski, J., Lok, S., Plavsic, N., and Chou, J. L., 1989. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science v. 245 p. 1066–1073. Wang, X. F., et al., 2003. Involvement of CFTR in uterine bicarbonate secretion and the fertilizing capacity of sperm. Nat. Cell Biol. v. 5 p. 902–906.