Indice di archiviazione TMF/ISF per studi clinici V3.0
advertisement
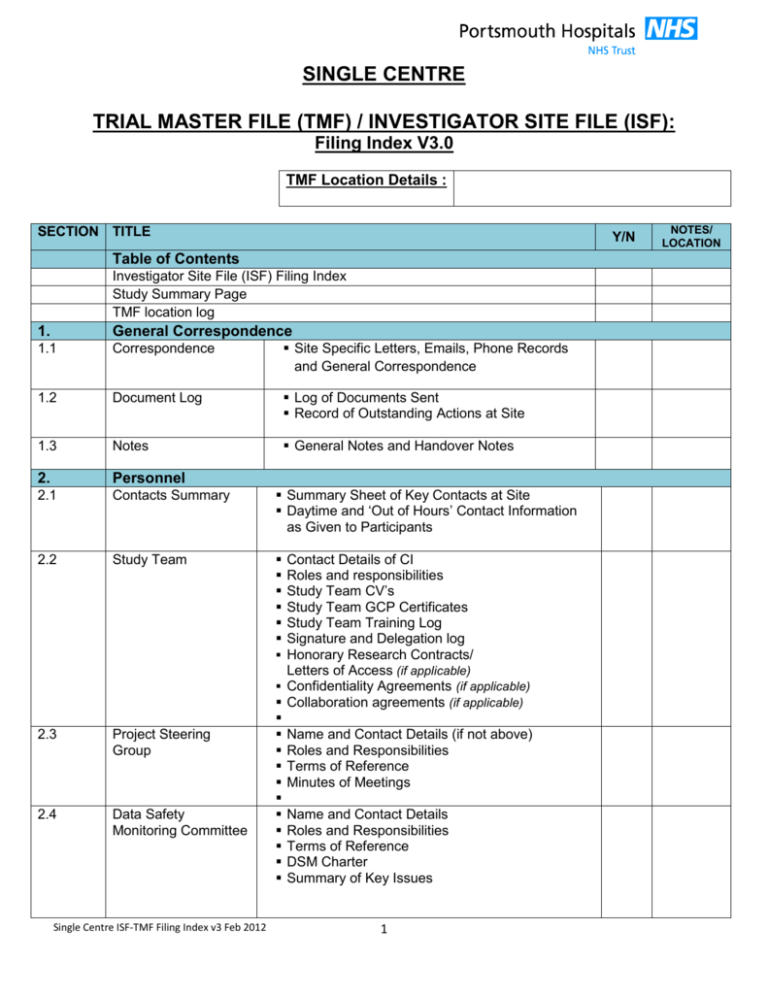
SINGLE CENTRE TRIAL MASTER FILE (TMF) / INVESTIGATOR SITE FILE (ISF): Filing Index V3.0 TMF Location Details : SECTION TITLE Y/N Table of Contents Investigator Site File (ISF) Filing Index Study Summary Page TMF location log 1. General Correspondence 1.1 Correspondence Site Specific Letters, Emails, Phone Records and General Correspondence 1.2 Document Log Log of Documents Sent Record of Outstanding Actions at Site 1.3 Notes General Notes and Handover Notes 2. Personnel 2.1 Contacts Summary Summary Sheet of Key Contacts at Site Daytime and ‘Out of Hours’ Contact Information as Given to Participants 2.2 Study Team 2.3 Project Steering Group 2.4 Data Safety Monitoring Committee Single Centre ISF-TMF Filing Index v3 Feb 2012 Contact Details of CI Roles and responsibilities Study Team CV’s Study Team GCP Certificates Study Team Training Log Signature and Delegation log Honorary Research Contracts/ Letters of Access (if applicable) Confidentiality Agreements (if applicable) Collaboration agreements (if applicable) Name and Contact Details (if not above) Roles and Responsibilities Terms of Reference Minutes of Meetings Name and Contact Details Roles and Responsibilities Terms of Reference DSM Charter Summary of Key Issues 1 NOTES/ LOCATION SECTION TITLE Y/N 3. Sponsorship, Agreements & Funding 3.1 Sponsorship & Agreements Written Letter/Agreement from Research Governance Sponsor Details of Co-Sponsorship Arrangements or Delegation of Roles (if applicable) Contractual Arrangements with Co-Sponsor Organisations (if applicable) Signed (Clinical Trial) Agreement between Sponsor & Site (if applicable) Indemnity Arrangements Indemnity Certificates for Non-NHS Organisations Signed Confidentiality Agreement between Sponsor and Site (if applicable) Other Agreement with Sponsor (e.g. Material Transfer) Correspondence 3.2 Funding 4. Supplies 4.1 Suppliers NOTES/ LOCATION Written Agreement from Funding Body Grant award Grant application Correspondence Contact Details of Supplier/s Contracts or Written Agreement with Supplier/s (Supply agreement) Procurement Arrangements Records of Receipt, Retention & Returns Current QP ‘Certificate of Analysis’ for shipped IMP/product Marketing Authorisation IMP license for supplier of IMP Calibration Certificates Indemnity Certificates if Applicable/Warrenty 4.2 General Equipment and Supplies 5. Services 5.1 IMP/Product/ Intervention Management Single Centre ISF-TMF Filing Index v3 Feb 2012 Procurement details and order numbers Indemnity statement for equipment on loan Correspondence Details of Service Written Authorisations for Roles & Responsibilities Sub-Contracts (where an service is external) Financial Agreements Current Licence / Accreditation Certificates CVs, GCP & Training Certificates Training Logs 2 Maybe on SSIF (e.g. Pharmacy) SECTION TITLE 5.1 Product/IMP Services continued 6. Services 6.2 Body Fluids/Tissue (if applicable) 6.3 Site Tissue Bank (if applicable) Y/N Signature & Delegation Log (if separate log required) IMP/product Accountability log Product / IMP Shipping Log (if applicable) Product/ IMP Destruction Log (if applicable) Temperature Logs (if applicable) Record of Recalled Site Product / IMP Contact Details of Service Written Authorisations for Roles & Responsibilities Sub-Contracts/MTA (where service is external) Financial Arrangements Current License and Accreditation Certificates Current Normal Value Ranges Signature & Delegation Log (if separate log required) CVs, GCP & Training Certificates Training Logs Body Fluids/Tissue Accountability Log Body Fluids/Tissue Shipping Log (if applicable) Body Fluids/Tissue Destruction Log (if applicable) Contact Details of Service Written Authorisations for Roles & Responsibilities Sub-Contracts (where service is external) CVs, GCP & Training Certificates Training Logs Current License and Accreditation Certificates ARSAC certificates IRMER approvals Record of All Scans/Tests at Site Signature & Delegation Log (if separate log required) Other Services (e.g. PET) 7. Protocol and Associated Study Documents 7.1 7.2 Version Control Protocol Record of Approved Documents Current, Approved Version: Signed by CI & PI Randomisation procedure 7.3 Correspondence Documents (Templates & Single Centre ISF-TMF Filing Index v3 Feb 2012 Maybe on SSIF (e.g. Pathology) Contact Information for Tissue Bank & DI Material Transfer Agreement (if external) Current License for Tissue Bank 6.4 Localised Versions) NOTES/ LOCATION Current Participant Information Sheet Current Consent Form Current GP Letter Current Advertisements Other Approved Correspondence Documents 3 Maybe on SSIF (e.g. PET, Radiology) SECTION TITLE Y/N 7.4 Data Collection Tools Questionnaires Case Report Forms (CRFS) 7.5 Product/ Intervention / IMP (if applicable) 7.6 Other Documents Latest Investigator Brochure (IB, SmPC, PI) CE Mark Devices product manual Current, Approved, Version-Controlled Sample Label for Medical Records Showing Patients’ Involvement in the Study 8. Standard Operating Procedures (SOPS) and Template Logs 8.1 Participant Logs Site Participant Screening Log (Template) Site Participant Enrolment Log (Template) 8.2 Study Team Logs Delegation & Authorisation Log (Template) Training Log (Template) 8.3 Product/Intervention / IMP SOPS Manuals and SOPS Approved, version-controlled labels attached to Pharmacy SOPs Sample Logs 8.4 Bodily Fluids / Tissue SOPS Study Laboratory Manual Sample Logs Sample labels for Pathology Tests Pathology SOPS 8.5 Radiology SOPS 8.6 Safety SOPS 8.7 Other Study SOPS 9. Regulatory Approval 9.1 MHRA (CTIMPS & Device Trials Only) Site Radiology Manual Scan Request Forms or Labels Radiology SOPS Study Safety Reporting Procedure AE/SAE Forms AE Logs Emergency Decoding/Unblinding Procedure Approval Letter Clinical Trial Application (CTA)/IRAS Annual Report 9.2 REC Approval Letter Main REC form / IRAS Annual Report 9.3 NHS Permissions R&D Form (IRAS) Conditions of Approval Site Specific Information Form (SSIF) Governance Report (if applicable) Single Centre ISF-TMF Filing Index v3 Feb 2012 4 NOTES/ LOCATION SECTION TITLE Y/N Approval Letter Main Form / IRAS Annual Report Risk Risk Assessment/s 10.2 Site Initiation Record of Site Initiation Visit/ checklist Site Initiation Report Correspondence/Follow-up Letters/Actions 10.3 Site Monitoring Approved Monitoring Plan Record of Site Monitoring Visit Monitoring Reports and Action Plan Monitoring Action Log Correspondence/Follow-up Letters Site Close Out Record of Site Close Out Visit/ checklist 9.4 GTAC/OTHER 10. Monitoring 10.1 11. Participant Recruitment 11.1 Participants 11.2 Randomisation Master Randomisation list (Blind maintained) Master ID code list (if appropriate) 12. Data Collection 12.1 Records/Logs 12.2 Data Analysis 13. Safety Reports 13.1 Reports 13.2 Annual Reports 13.3 13.4 Master Reports Expedited Reports 13.5 Notifications Single Centre ISF-TMF Filing Index v3 Feb 2012 Participant Screening Log Participant Recruitment Log Participant ID Code List Participant Transfer Records Participant Consent Forms Record of source data (where it can be found) Completed Data Collection Forms (CRF’S) Analytical workings Summary reports Excel spreadsheets Notes SAE/SSAR/SUSAR Initial Reports SAE/SUSAR Follow-Up Reports Correspondence Record of Annual Safety Report(s) Due Date(s) MHRA /REC Annual Safety Reports (DSURS) AE Line Listing SUSAR Reports to MHRA and REC SAE Reports to REC Safety notifications to & from Suppliers 5 NOTES/ LOCATION SECTION TITLE 14. Finance 14.1 Financial Records 15. Amendments 15.1 Y/N Study Finance Schedule Expenditure Plan Invoices Payment Records Study cost centre details Log Log of Amendments and Dates 15.2 Amendments Amendment Details Filed in Chronological Order to Include all Paperwork Submitted for Regulatory Approval 16. Superseded Documents 16.1 16.2 16.3 16.4 Project Management Personnel Protocol Documents Sponsorship & Funding SOPS & Logs Regulatory Approvals Site Services Finance Other 16.5 16.6 16.7 16.8 16.9 Single Centre ISF-TMF Filing Index v3 Feb 2012 Superseded Documents–Section 2 Superseded Documents - Section Superseded Documents – Section 3 Superseded Documents – Section Superseded Documents–Section 4 Superseded Documents–Section 5 Superseded Documents–Section 7 Superseded Documents–Section 11 6 NOTES/ LOCATION
