Endothermic & Exothermic Reactions Lab Worksheet
advertisement
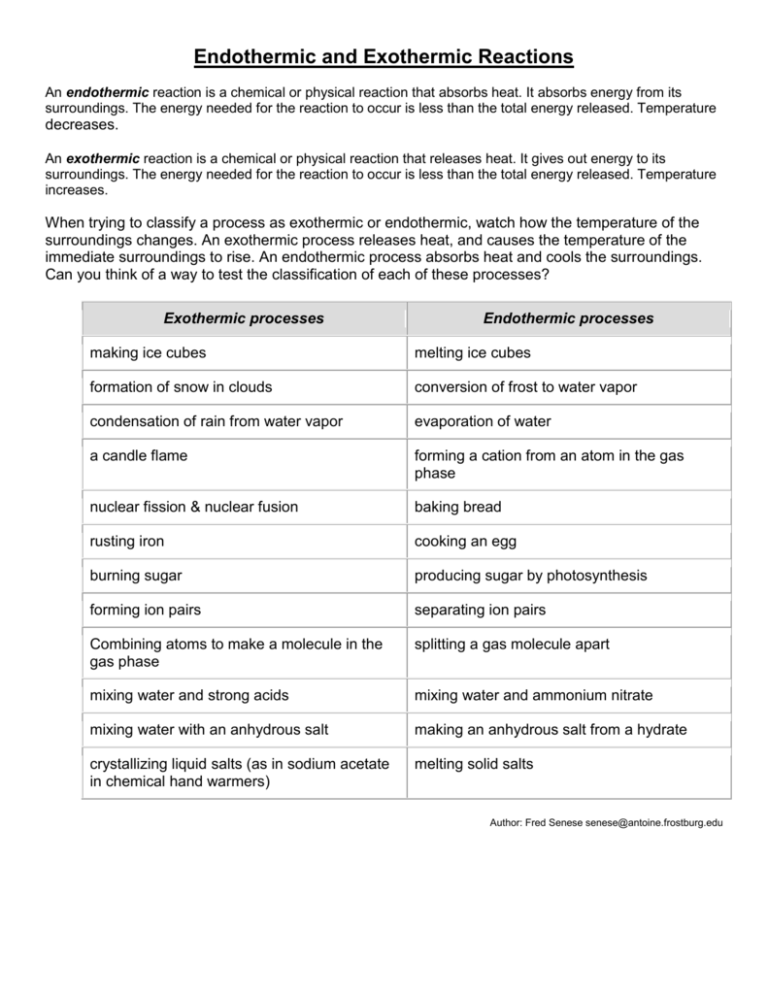
Endothermic and Exothermic Reactions An endothermic reaction is a chemical or physical reaction that absorbs heat. It absorbs energy from its surroundings. The energy needed for the reaction to occur is less than the total energy released. Temperature decreases. An exothermic reaction is a chemical or physical reaction that releases heat. It gives out energy to its surroundings. The energy needed for the reaction to occur is less than the total energy released. Temperature increases. When trying to classify a process as exothermic or endothermic, watch how the temperature of the surroundings changes. An exothermic process releases heat, and causes the temperature of the immediate surroundings to rise. An endothermic process absorbs heat and cools the surroundings. Can you think of a way to test the classification of each of these processes? Exothermic processes Endothermic processes making ice cubes melting ice cubes formation of snow in clouds conversion of frost to water vapor condensation of rain from water vapor evaporation of water a candle flame forming a cation from an atom in the gas phase nuclear fission & nuclear fusion baking bread rusting iron cooking an egg burning sugar producing sugar by photosynthesis forming ion pairs separating ion pairs Combining atoms to make a molecule in the gas phase splitting a gas molecule apart mixing water and strong acids mixing water and ammonium nitrate mixing water with an anhydrous salt making an anhydrous salt from a hydrate crystallizing liquid salts (as in sodium acetate in chemical hand warmers) melting solid salts Author: Fred Senese senese@antoine.frostburg.edu Endothermic Reaction – Demonstration #1 Materials: 32g OR 6g of barium hydroxide octahydrate 17g OR 3g ammonium thiocyanate (or you could use ammonium nitrate or ammonium chloride) 125-ml flask OR test tube stirring rod Procedure: Pour the barium hydroxide and ammonium thiocyanate into the flask. Stir the mixture. The odor of ammonia should become evident within about 30 seconds. If you hold a piece of dampened litmus paper over the reaction you can watch a color change showing that the gas produced by the reaction is basic. Liquid will be produced, which will freeze into slush as the reaction proceeds. If you set the flask on a damp block of wood or piece of cardboard while performing the reaction you can freeze the bottom of the flask to the wood or paper. You can touch the outside of the flask, but don't hold it in your hand while performing the reaction. After the demonstration is completed, the contents of the flask can be washed down the drain with water. Do not drink the contents of the flask. Avoid skin contact. If you get any solution on your skin, rinse it off with water. Endothermic Reaction – Demonstration #2 Materials: 25 ml citric acid soln 15 g baking soda styrofoam cup thermometer stirring rod Procedure: 1. Pour the citric acid solution in a styrofoam coffee cup. Use a thermometer or other temperature probe to record the initial temperature. 2. Stir in the baking soda (sodium bicarbonate). Track the change in temperature as a function of time. 3. The reaction is: H3C6H5O7(aq) + 3 NaHCO3(s) → 3 CO2(g) + 3 H2O(l) + Na3C6H5O7(aq) 4. When you have completed your demonstration or experiment, simply wash the cup out in a sink. No toxic chemicals to mess with! Tips: 1. Feel free to vary the concentration of the citric acid solution or the quantity of sodium bicarbonate. 2. An endothermic is a reaction that requires energy to proceed. The intake of energy may be observed as a decrease in temperature as the reaction proceeds. Once the reaction is complete, the temperature of the mixture will return to room temperature. Exothermic Reaction – Demonstration #1 Materials: blast shield Large water jug - (can get from water company) Methanol Lit match on end of tongs Procedure: Swirl small amount of methanol in jug ( approx. 5-7 ml) Roll jug to disperse and vaporize methanol. Pour out any liquid remaining after spinning jug a few times. Turn out lights - ignite with match held with tongs. Exothermic Reaction – Demonstration #2 Gummy Bear Sacrifice Description: Potassium chlorate is such a strong oxidizer that when a gummy bear, which is mostly sugar, is placed into it, the sugar is oxidized violently producing noise and a flame. This demo can be used to discuss strong oxidizers as well as how much energy is in sugar. The reactions proceed as follows: Materials: potassium chlorate gummy bears or worms test tube (needs to have a large enough diameter to fit the candy) ring stand and clamp that holds the test tube tweezers hot plate or Bunsen burner or ethanol burner Procedure: Place a small amount of KClO3 into the test tube (~1/4 inch of the test tube) Position test tube, in the clamp, over the heat source until the KClO3 melts (open flames melt it much faster than hot plates) Remove the heat source and turn it off. Carefully use the tweezers to place ½ a worm or a whole bear into the test tube Safety: Goggles should always be worn, and the reaction should be done in a fume hood. Exothermic Reaction – Demonstration #3 Material: Thermometer Jar with Lid Steel Wool Vinegar Procedure: 1. Place the thermometer in the jar and close the lid. Allow about 5 minutes for the thermometer to record the temperature, then open the lid and read the thermometer. 2. Remove the thermometer from the jar (if you didn't already in Step 1). 3. Soak a piece of steel wool in vinegar for 1 minute. 4. Squeeze the excess vinegar out of the steel wool. 5. Wrap the wool around the thermometer and place the wool/thermometer in the jar, sealing the lid. 6. Allow 5 minutes, then read the temperature and compare it with the first reading. Tips: 1. Not only does the vinegar remove the protective coating on the steel wool, but once the coating is off its acidity aids in oxidation (rust) of the iron in the steel. 2. The thermal energy given off during this chemical reaction causes the mercury in the thermometer to expand and rise up the column of the thermometer tube. 3. In the rusting of iron, four atoms of solid iron react with three molecules of oxygen gas to form two molecules of solid rust (iron oxide). __ 25 Name: _______________________________ Endothermic and Exothermic Reaction Lab Every chemical reaction that exists is one of two things: endothermic or exothermic. The Greek root therm means temperature or heat, which gives us a clue about all reactions: there is energy exchange! Endo means "within" while exo means "outside," so these types of reactions are opposite. Endothermic reactions are those which absorb heat during the reaction. They take in more energy than they give off, which leaves the surroundings cooler than the starting point. Evaporation of water by sunlight is a great example. The sun and the liquid water combine and the water absorbs energy eventually becoming a gas. Exothermic reactions are exactly the opposite. While they take some energy to get going, called the activation energy of reaction, these reactions give off heat during the reaction. Good examples of exothermic reactions are explosions such as fireworks and the burning of fuel in a combustion engine. Problem: Observe endothermic and exothermic reactions and determine which absorb or produce the most energy. Which reactions will be endothermic? Which reactions will be exothermic? Materials: Beaker, test tube Water White vinegar Hydrogen Peroxide Sodium bicarbonate Magnesium sulfate Sodium hydroxide Ammonium nitrate Ammonium chloride Calcium chloride Sodium chloride Dry yeast Thermometer Safety Goggles (worn at all times) Procedure: 1. The table below has a list of solids to combine with liquids. Proceed to a station and follow the directions at the station for the appropriate amounts. 2. Fill beaker or test tube with indicated amount. 3. Record the initial temperature of the liquid on the provided table. 4. Measure the correct amount of solid and add to liquid. 5. Wait 2 minutes and then record the final temperature of the solution on the provided table. Note: It is important to measure the same time for each trial as time is the control variable. 6. Discard the solution, clean the beaker or test tube and proceed to the next combination. 7. For each trial, calculate the change in temperature of the reaction by subtracting the final value from the initial value. Observations and Data: Liquid Additive 50ml of water 25g MgSO4 – magnesium sulfate ¼ test tube of water ½g NaOH – sodium hydroxide 50ml of water 10g NH4NO3 – ammonium nitrate 50ml of water 10g NaCl – sodium chloride 50ml of water 10g NH4Cl – ammonium chloride 50ml of water 5g CaCl2 – calcium chloride 25ml of white vinegar 5g NaHCO3 – sodium bicarbonate 25ml Hydrogen Peroxide 3g – dry yeast Ti Conclusion: Define endothermic and exothermic in your own words. Which reactions were endothermic? Which reactions were exthothermic? Classify each of the following as an exothermic or endothermic process. Melting ice cubes ________________ Burning a candle ________________ Evaporation of water ________________ Baking Bread ________________ Splitting a gas molecule apart ________________ Formation of snow in clouds ________________ Tf ΔT Endothermic or Exothermic








