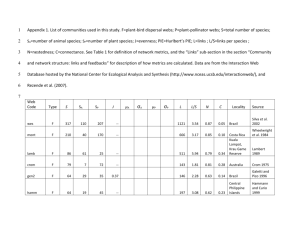
Plant-animal pollination interaction networks in Australia
advertisement

Plant-animal pollination interaction networks in Australia Project Summary Mutualistic interactions are important in structuring communities but their role may have been underestimated compared to competition and predation. Recent studies have shown that pollination is a predominantly generalised interaction, and so interacting species exist within a network. These networks display properties of asymmetry, nestedness, high connectance and strong small-world properties. Evidence for this new paradigm has been gathered from a variety of habitats, with few contributions from Australia and arid systems. Australian arid systems are of interest due to the suggestion that positive interactions are more likely in harsh environments, and possess potentially unaltered plant – pollinator networks due to the absence of exotic species. The aim of this study is to understand the main interaction network of plants and their floral visitors in a variety of Australian habitats especially arid environments. Aims and Significance of the Project Interactions are of direct importance in shaping terrestrial communities and in the maintenance of biodiversity. Empirical evidence suggests both competition (Fowler 1986; Goldberg & Novoplansky 1997) and facilitation (Bertness & Callaway 1994; Bruno et al. 2003) are important; but the balance between these two is unresolved. Mutualistic interactions are relationships between two species that result in reciprocal benefits (Pellmyr 2002). Such interaction are particularly important for flowering plant communities (Brooker et al. 2008), as for instance, flowering plants depend on pollinator mutualists for reproduction. Such mutualisms are ubiquitous in nature: over 90% of the 240 000 flowering plant species rely on animal pollinators to reproduce (Buchmann & Nabhan 1996), and the number of pollinator species is predicted to be up to 300 000 (Shepherd et al. 2003). Pollination is directly related to the supply of food for animals as seed, or directly from consuming nectar and/or pollen (Pellmyr 2002). However, our understanding of the role of mutualistic interactions play in shaping terrestrial communities is less than that for other interactions such as competition and predation (Bruno et al. 2003; Bascompte & Jordano 2007). Thus, such factors are rarely incorporated into models of factors that impact populations and communities (Stachowicz 2001). The importance of indirect interactions in shaping the diversity and evolution of plant communities has also been neglected; despite evidence such processes are important and widespread (Brooker et al. 2008; Sargent & Ackerly 2008). Pollination ecology is the study of how plants receive and donate pollen, including floral phenolgy (seasonal timing of biological events), floral adaptation, and animal behaviour (Davila 2006). Pollen transport can be mediated either by abiotic (wind and water) or biotic (animal) vectors. To attract animal pollinators, plants have evolved a range of visual, olfactory and auditory cues to signal the presence of a reward, the most common being nectar and pollen (Pellmyr 2002). Rewards such as nectar have been shown to influence the assemblage of visitors to a plant, and also to a community (Potts et al. 2004). Floral visitors are not necessarily pollinators; they may be consuming rewards such as nectar or pollen (Pellmyr 2002; Michener 2007; Zhang et al. 2007), consuming flowers (florivore) (McCall & Irwin 2006), or preying on other visitors. A visitor becomes a pollinator when transporting pollen from the anther to the stigma of a flower (Pellmyr 2002). The effectiveness of pollinators can vary depending on quality and quantity of pollen deposited (Gibson et al. 2006; Lopezaraiza-Mikel et al. 2007). The study of plant-pollinator interactions has tended to focus on direct pair-wise interactions (Sargent & Ackerly 2008), where both plant and animal species are specialists: a plant species that is pollinated by one animal species, and vice versa. This is driven by the view that plant-pollinator interactions evolve towards specialisation such as those between Ficus and the fig wasp (Michaloud et al. 1996). However, there is now substantial evidence to suggest that plant-pollinator interactions are indeed generalised; that is, a given plant species is pollinated by many animal species and an animal species pollinates many plant species (Waser et al. 1996; Olesen 2000). 1 As pollination is a predominantly generalised interaction, indirect and direct interactions are pervasive in a community of plants and their pollinators. By studying pair-wise interactions exclusively, the role that direct and indirect interactions play in influencing an organism’s abundance, phenotypes and genotypes has been largely neglected (Strauss & Irwin 2004). Rather, we can study the network of plant-pollinator interactions in the manner of conventional food webs (Memmott 1999). As a result, we can investigate how ecological and evolutionary processes organise communities of plants and their pollinators which allows us to identify mechanisms behind the persistence of biodiversity (Bascompte & Jordano 2007). Identifying mechanisms behind biodiversity persistence is still an area of interest in general ecology (Bruno et al. 2003; Proulx et al. 2005; Bascompte & Jordano 2007; Brooker et al. 2008). To date, studies of plant-pollinator networks have revealed patterns of nestedness (generalist species interacting amongst each other, and specialist species interacting only with generalists) (Bascompte et al. 2003; Memmott et al. 2004; Bascompte & Jordano 2007), asymmetry of dependencies (few interactions where a species will depend heavily on another species, and many interactions where dependencies are weak) (Bascompte et al. 2006), species connectivity distributions with a power-law regime (few plants with many interactions and many plants with few interactions) (Jordano et al. 2003; Memmott et al. 2004; Vazquez 2005), and very strong smallworld properties (all species are close to each other and highly clustered) (Olesen et al. 2006a). These structural attributes have consequences for the resilience of the plant-pollinator communities (Memmott et al. 2004; Bascompte & Jordano 2007). For instance, connectivity distributions with a power-law regime give the community heightened resilience to random extinctions (Albert & Barabasi 2000). Such characteristics have been found from pollinator networks of predominantly temperate, tropical and alpine environments !!!!!!!!! Olesen & Jordano (2002) Olesen et al. (2006b). Recently, a study into the pollinator network in an Australian arid habitat has revealed similar structural patterns (Popic et al, in prep), despite the environment being of unpredictable ‘boom-bust’ cycles contrasting to the predictability of other environments. Furthermore, the network was rich in species and complex in makeup, contradicting the common belief that arid environments are of extremely low productivity (Knox et al. 2001). As 70% of the Australian continent is classified as arid or semi-arid, and this level is increasing due to anthropogenic factors, a greater understanding of communities in such environments can be applied to mitigate the impacts from environmental problems such as climate change and land degradation. The few pollinator networks to be sampled in Australia have all featured the introduced ‘super-generalist’ Apis mellifera, except for a single pollinator network from Spinifex-dominated grassland in the Simpson Desert (Popic et al, in prep). This network was rich in diversity of small solitary native bees, suggesting such animals are important pollinators in arid Australia. This is in disagreement with the prevailing paradigm that birds are the major pollinators of arid Australia (Ford et al. 1979; Keighery 1982; Orians & Milewski 2007). In addition to the role and general importance of bees being unknown, Australia’s bee fauna is still relatively unknown (Michener 1979, 2007). Investigations into non-invaded plant-pollinator communities are required in order to understand impacts when they are altered. Plant phenology is always variable but is expected to change rapidly with climate change, leading to altered networks (Memmott et al. 2007). The impact of introduced species, both plants (Lopezaraiza-Mikel et al. 2007; Bartomeus et al. 2008) and visitors such as A. mellifera and attempts to establish Bombus terrestris (Hingston 2006) in mainland Australia, can only be fully known with an understanding of plant – pollinator networks before they are altered. In this study, the broad aim is to understand interaction networks of plants and their visitors in different Australian environments, particularly arid environments where they are still largely unaltered. It will contain the components: plant phenology, plant – visitor networks, pollen 2 transport networks, effective pollination by pollen transporters, floral attractants and rewards and indirect interactions. Plant phenology (seasonal timing of biological events), along with the diversity and abundance of potential pollinators dictate what interactions are possible. Investigating flowering phenology will establish the potential for indirect interactions between plants through their visitors and between visitors through the plants. For instance, two co-flowering plants can either increase each other’s reproductive success by facilitating each other’s pollination, decrease each other’s reproductive output by competing for pollinators or have no effect on each other. Visitation webs identify the interactions taking place between flowering plants and animal visitors, and provides a tool to study the way interactions are assembled. Network structure can be influenced by flowering phenology, floral attractants and rewards and the diversity and abundance of visitors. A variety of different environments will be sampled to determine difference in structure between plant – pollinator communities. Commonalities in plant-pollinator networks from different environments suggest common structural mechanisms shaping plant-pollinator communities. Such mechanisms may be both evolutionary and ecological ones (ref) and such mechanisms will be investigated. By sampling a range of environments, the importance of different visitor types in different environments will also be investigated. Areas of arid Australia are free from exotic insects such Apis mellifera (Popic et al, in prep) and are rich in native bees which dominate plant-pollinator networks. Sampling these communities in conjunction with invaded communties, allows us to investigate the impacts on pollinator networks by exotics such as Apis mellifera. Determining the type and strength of interactions between species in a plant-pollinator network is important in order to determine the relative importance of mutualisms. The first step is to identify pollen transporters. The insect pollen transport web will determine which visitors are likely pollinators by quantifying which insects move which pollen. Next we can begin detailed observations and pollinator exclusion experiments (inouye book ref***) to determine which visitors are importance pollinators. Determining effective pollinators from pollen transporters will also determine the effectiveness of using pollen transport as an indication of pollination. It will also give a better indication of the type of interaction between plants and their visitors. As plant – pollinator networks are highly generalised and small world, constituents within the networks are interacting indirectly. For instance, plant species could be supporting pollinator assemblages collectively, attracting a greater diversity and abundance of pollinators that could increase seed output, and thereby indirectly facilitating each others pollination (ref!!!sargent. Research Plan, Methods and Technique Study sites The arid zone study areas will include the reserves of Cravens Peak (23 S, 138 E) and Ethabuka (23 S, 138 E) and the station of Carlo (23 S, 138 E), an area in the North Eastern Simpson Desert, South Western Queensland. The dominant landforms are long parallel sand dunes, 8-10m in height, divided by interdune valleys (swales). Triodia basedowii (spinifex) dominate swales and dune sides and common shrubs include Grevillea stenobotrya, Eremophila spp and Acacia spp. Some swales also have clay soils with the tree Acacia cambagei. Dune crests commonly feature G. stenobotrya and Eremophila spp. Many ephemeral forbs and herbs are present after rain. Over 150 plant species, seven native small mammal species, 42 reptile species and an unknown number of invertebrate species exist in the area. Other arid environments will be chosen according to recent rain events, as rain promotes flowering in Arid Australia (ref). Other environments may include, and are not limited to heath, mallee woodlands, open woodlands and sclerophyllus forests. Plant Phenology Data on the flowering and seed production for all plant species will be collected across a spatial and temporal time scale using sampling plots. A schematic portrayal of these data will be 3 Spp generated in MS Excel (Figure 1) to show how long plants flower, what species co-flower and long they co-flower for. Time Figure 1: Plant phenology diagram. Black represents flowering periods, white represents fruiting. Insect visitation web Data to construct visitation webs will be collected by catching floral insect visitors (observations will also be used if the visitor can be identified) using both plots and transects. Ten minute catching periods in 2x2m plots will be used to sample visitors of common flowering plants, whereas a 2m wide 100m transect will be used to sample the less common flowering plants. All visitors will be sampled. Sampling will also occur opportunistically while in the field (these two catching protocols will be used to generate different visitation webs). Insects will be caught either using a net, pooter or directly into a vial, and killed using a killing jar containing ethyl acetate. Insects collected will be differentiated to morphospecies for the purpose of web construction and sent to experts at the Australian Museum for further resolution. A visitation web will be constructed in Excel. Power-law distribution will be tested using a Kolmogorov-Smirnov test in R. To detect structure such as nestedness and compartments, assemblages will be arranged into binary (presence/absence of interaction) matrices and tested with Aninhado software (Guimaraes Jr & Guimaraes 2006). Network connectance, average path length and mean number of interactions across animal and plant species will be calculated in the program Pajek. These network parameters will be used to compare desert sand dune visitor assemblages to those from other environments. Networks generated on different trips will also be compared to determine temporal variation of structure. Insect Pollen Transport Web Visitors will be used to construct an insect pollen transport web. Insects will be systematically dabbed with a 20mm3 section of gelatin-fuchsin to sample and stain pollen. Pollen storage areas such as pollen baskets will be avoided as these contain pollen unlikely to be available for pollination (Gibson et al. 2006). The gel will then be placed on a microscope slide, heated to melting point then covered with a cover slip. Pollen will be identified under a light microscope using a pollen reference set. Pollinator importance (PI), a measure of the importance of a particular insect species in pollinating a particular plant species (Schemske & Horvitz 1984), will be determined for the bees sampled. A modified version from Gibson et al.(2006) will be used: PI = (relative abundance of pollinator) X (pollen fidelity) For instance, to calculate the PI of insect α for plant β, the proportion of all insects carrying β pollen that are of species α, would be multiplied by the mean proportion of individual α pollen loads that originate from β. A web will be constructed which will take into account the pollinator importance as in Gibson et al.(2006). Networks will be analysed as described previously. Determining Pollinators For certain plant species the relationship between it and its visitors will be determined. Determining effective pollination includes pollen pickup and transport to a conspecific stigma, and experiments excluding different visitors so as determine the importance of a given species (Jennersten 1988; Mayfield et al. 2001; Pellmyr 2002). 4 Floral Rewards Floral attractants and rewards can influence the structure of plant – visitor assemblages (potts…) !!!!!Floral rewards usually come in two forms: nectar and pollen. Nectar can be quantified using capillary tubes, and qualified using a refractometer. Pollen will be assessed by amount present through collection and counting. Indirect Interactions Indirect relationships between plants via common pollinators can be investigated by looking at the reproductive output of plants in different local plant communities, in conjunction with experimentally setup arrays. References Albert R. & Barabasi A. (2000). Topology of evolving networks: local events and universality. Physical review letters, 85, 5234-5237. Bartomeus I., Vila M. & Santamaria L. (2008). Contrasting effects of invasive plants in plantpollinator networks. Oecologia, 155, 761-770. Bascompte J. & Jordano P. (2007). Plant-animal mutualistic networks: The architecture of biodiversity. Annual Review of Ecology Evolution and Systematics, 38, 567-593. Bascompte J., Jordano P., Melian C.J. & Olesen J.M. (2003). The nested assembly of plant-animal mutualistic networks. Proceedings of the National Academy of Sciences of the United States of America, 100, 9383-9387. Bascompte J., Jordano P. & Olesen J.M. (2006). Asymmetric coevolutionary networks facilitate biodiversity maintenance. Science, 312, 431-433. Bertness M.D. & Callaway R. (1994). Positive interactions in communities. Trends in Ecology & Evolution, 9, 191-193. Brooker R.W., Maestre F.T., Callaway R.M., Lortie C.L., Cavieres L.A., Kunstler G., Liancourt P., Tielborger K., Travis J.M.J., Anthelme F., Armas C., Coll L., Corcket E., Delzon S., Forey E., Kikvidze Z., Olofsson J., Pugnaire F., Quiroz C.L., Saccone P., Schiffers K., Seifan M., Touzard B. & Michalet R. (2008). Facilitation in plant communities: the past, the present, and the future. Journal of Ecology, 96, 18-34. Bruno J.F., Stachowicz J.J. & Bertness M.D. (2003). Inclusion of facilitation into ecological theory. Trends in Ecology & Evolution, 18, 119-125. Buchmann S.L. & Nabhan G.P. (1996). The forgotten pollinators. Island Press/Shearwater Books, Washington D.C. Davila Y.C. (2006). Pollination Ecology of Trachymene incisa (Apiaceae): Understanding generalised plant-pollinator systems. In. The University of Sydney. Ford H.A., Paton D.C. & Forde N. (1979). Birds as pollinators of Australian plants. New Zealand Journal of Botany, 17, 509-519. Fowler N. (1986). The role of competition in plant communities in arid and semiarid regions. Annual Review of Ecology and Systematics, 17, 89-110. Gibson R.H., Nelson I.L., Hopkins G.W., Hamlett B.J. & Memmott J. (2006). Pollinator webs, plant communities and the conservation of rare plants: arable weeds as a case study. Journal of Applied Ecology, 43, 246-257. Goldberg D. & Novoplansky A. (1997). On the relative importance of competition in unproductive environments. Journal of Ecology, 85, 409-418. Guimaraes Jr P.R. & Guimaraes P. (2006). Improving the analyses of nestedness for large sets of matrices. Environmental Modelling & Software, 21, 1512-1513. Hingston A.B. (2006). Is the exotic bumblebee Bombus terrestris really invading Tasmanian native vegetation? Journal of Insect Conservation, 10, 289-293. Jennersten O. (1988). Pollination in Dianthus deltoides (Caryophyllaceae): Effects of habitat fragmentation on visitation and seed set. Conservation Biology, 2, 359-366. 5 Jordano P., Bascompte J. & Olesen J.M. (2003). Invariant properties in coevolutionary networks of plant-animal interactions. Ecology Letters, 6, 69-81. Keighery G.J. (1982). Bird-pollinated plants in Western Australia. In: Pollination and Evolution (eds. Armstrong JA, Powell JM & Richards AJ). Royal Botanic Gardens Sydney. Knox R.B., Ladiges P., Evans B. & Saint R. (2001). Biology. McGraw-Hill Book Company Australia, Roseville. Lopezaraiza-Mikel M.E., Hayes R.B., Whalley M.R. & Memmott J. (2007). The impact of an alien plant on a native plant-pollinator network: an experimental approach. Ecology Letters, 10, 539-550. Mayfield M.M., Waser N.M. & Price M.V. (2001). Exploring the 'most effective pollinator principle' with complex flowers: Bumblebees and Ipomopsis aggregata. Annals of Botany, 88, 591-596. McCall A.C. & Irwin R.E. (2006). Florivory: the intersection of pollination and herbivory. Ecology Letters, 9, 1351-1365. Memmott J. (1999). The structure of a plant-pollinator food web. Ecology Letters, 2, 276-280. Memmott J., Craze P.G., Waser N.M. & Price M.V. (2007). Global warming and the disruption of plant-pollinator interactions. Ecology Letters, 10, 710-717. Memmott J., Waser N.M. & Price M.V. (2004). Tolerance of pollination networks to species extinctions. Proceedings of the Royal Society of London Series B-Biological Sciences, 271, 2605-2611. Michaloud G., Carriere S. & Kobbi M. (1996). Exceptions to the one:one relationship between African fig trees and their wasp pollinators: possible evolutionary scenarios. Journal of Biogeography, 23, 513-520. Michener C.D. (1979). Biogeography of the bees. Annals of the Missouri Botanical Garden, 66, 277-347. Michener C.D. (2007). The Bees of the World. Second edn. The John Hopkins University Press, Baltimore, Maryland. Olesen J.M. (2000). Exactly how generalised are pollination interactions? In: The Scandinavian Association for Pollination Ecology honours Knut Faegri (eds. Totland O, Armbruster WS, Fenster CB, Molau U, Nilsson LA, Olesen JM, Ollerton J, Philipp M & Agren J). The Norwegian Acadamy of Science and Letters Oslo, pp. 161-178. Olesen J.M., Bascompte J., Dupont Y.L. & Jordano P. (2006a). The smallest of all worlds: Pollination networks. Journal of Theoretical Biology, 240, 270-276. Olesen J.M., Bascompte J., Dupont Y.L. & Jordano P. (2006b). The smallest of all worlds: Pollination networks. Journal of Theoretical Biology, 240, 270-276. Olesen J.M. & Jordano P. (2002). Geographic patterns in plant-pollinator mutualistic networks. Ecology, 83, 2416-2424. Orians G.H. & Milewski A.V. (2007). Ecology of Australia: the effects of nutrient-poor soils and intense fires. Biological Reviews, 82, 393-423. Pellmyr O. (2002). Pollination by animals. In: Plant-Animal Interactions: An Evolutionary Approach (eds. Herrera CM & Pellmyr O). Blackwell Sciences Ltd Oxford, pp. 157-184. Potts S.G., Vulliamy B., Roberts S., O'Toole C., Dafni A., Ne'eman G. & Willmer P.G. (2004). Nectar resource diversity organises flower-visitor community structure. Entomologia Experimentalis Et Applicata, 113, 103-107. Proulx S.R., Promislow D.E.L. & Phillips P.C. (2005). Network thinking in ecology and evolution. Trends in Ecology & Evolution, 20, 345-353. Sargent R.D. & Ackerly D.D. (2008). Plant-pollinator interactions and the assembly of plant communities. Trends in Ecology & Evolution, 23, 123-130. Schemske D.W. & Horvitz C.C. (1984). Variation among floral visitors in pollination ability: a precondition for mutualism specialization. Science, 225, 519-521. Shepherd M., Buchmann S.L., Vaughan M. & Hoffman Black S. (2003). Pollinator Conservation Handbook. The Xerces Society, Portland. 6 Stachowicz J.J. (2001). Mutualism, Facilitation, and the Structure of Ecological Communities. Bioscience, 51, 235-246. Strauss S.Y. & Irwin R.E. (2004). Ecological and evolutionary consequences of multi-species plantanimal interactions. Annual Review of Ecology, Evolution and Systematics, 35, 435-466. Vazquez D.P. (2005). Degree distribution in plant-animal mutualistic networks: forbidden links or random interactions? Oikos, 108, 421-426. Waser N.M., Chittka L., Price M.V., Williams N.M. & Ollerton J. (1996). Generalization in pollination systems, and why it matters. Ecology, 77, 1043-1060. Zhang Y.W., Robert G.W., Wang Y. & Guo Y.H. (2007). Nectar robbing of a carpenter bee and its effects on the reproductive fitness of Glechoma longituba (Lamiaceae). Plant Ecology, 193, 1-13. 7