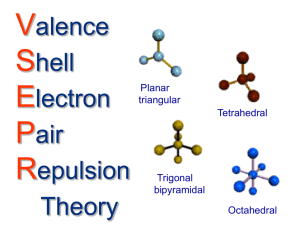
Irvington High School • AP Chemistry
advertisement

Irvington High School AP Chemistry Mr. Markic Name _______________________________ Number ___ Date ___/___/___ 10 Molecular Geometry & Bonding Theories Molecular Geometry 1. Predict the geometries of the following species using the VSEPR method: a. PCl3 c. SiH4 b. CHCl3 d. TeCl4 (a) The Lewis structure of PCl3 is shown below. Since in the VSEPR method the number of bonding pairs and lone pairs of electrons around the central atom (phosphorus, in this case) is important in determining the structure, the lone pairs of electrons around the chlorine atoms have been omitted for simplicity. There are three bonds and one lone electron pair around the central atom, phosphorus, which makes this an AB3E case. The information in Table 10.2 shows that the structure is a trigonal pyramid like ammonia. Cl P Cl Cl What would be the structure of the molecule if there were no lone pairs and only three bonds? (b) The Lewis structure of CHCl3 is shown below. There are four bonds and no lone pairs around carbon which makes this an AB4 case. The molecule should be tetrahedral like methane (Table 10.1). H Cl C Cl Cl (c) The Lewis structure of SiH4 is shown below. Like part (b), it is a tetrahedral AB4 molecule. H H Si H Page 1 of 3 H (d) The Lewis structure of TeCl4 is shown below. There are four bonds and one lone pair which make this an AB4E case. Consulting Table 10.2 shows that the structure should be that of a distorted tetrahedron like SF4. Cl Te Cl Cl Cl Are TeCl4 and SF4 isoelectronic? Should isoelectronic molecules have similar VSEPR structures? 2. Predict the geometry of the following molecules and ion using the VSEPR model: a. CBr4 d. H2Se e. NO2- b. BCl3 c. NF3 Lewis Structure e pair arrangement geometry tetrahedral tetrahedral trigonal planar trigonal planar tetrahedral trigonal pyramidal tetrahedral bent trigonal planar bent Br (a) Br C Br Br (b) Cl B Cl Cl (c) F N F F (d) (e) 3. Page 2 of 3 H Se O N H O Predict the geometry of the following molecules using the VSEPR model: a. HgBr2 b. N2O (arrangement of atoms is NNO) c. SCN- (arrangement of atoms is SCN) The lone pairs of electrons on the bromine atoms have been omitted for simplicity. Br 4. Hg Br linear N N O linear S C N linear Describe the geometry around each of the three central atoms in the CH3COOH molecule. H H C O C O H H O H H C AB4 tetrahedral H O Page 3 of 3 H AB2E2 bent C AB3 trigonal planar