Chapter 1 What is the difference between evidence and inference
advertisement

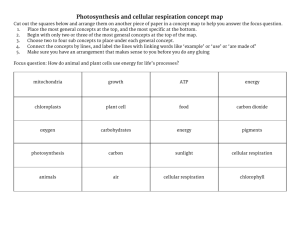
Chapter 1 What is the difference between evidence and inference? What is a testable question? How is a scientific investigation designed and conducted? Hypothesis, inference, evidence, testable question, homeostasis, carbohydrate, electrolytes, diuretics, control, scientific method, conductivity, distillation, theory, law Chapter 2 How is atomic structure related to physical properties? How is density related and not related to solubility? How do I determine density for solids and liquids? What are the basic building blocks of matter? How does a phase diagram represent states of matter? How is atomic structure related to physical properties? How is density related and not related to solubility? How do I determine density for solids and liquids? What are the basic building blocks of matter? How does a phase diagram represent states of matter? Macroscopic, microscopic, density, solubility, intensive and extensive properties, mass, atoms, elements, compound, molecule, pure substance, mixture, phases of matter, law of conservation of matter, phase diagrams Chapter 3 How do atoms interact due to forces between opposite charges? How do the interactions inside molecules result in different types of bonds? How do forces of interaction between molecules result in differences between physical properties? Insulator, conductor, electrical conductivity, conductivity meter, ions, ionic compound, crystal lattice, polar, dipole, covalent, solvation, ionization, dissociation, ionization energy, electronegativity, nonpolar Chapter 4 How do scientists infer atomic structure from indirect evidence? What are atoms made up of? How does atomic structure explain trends on the periodic table? Why do stable compounds and noble gases have similar electron configurations? How can logic, evidence, and critical analysis help select effective models? How are line spectra related to quanta of energy? How do electron dot diagrams represent valence electrons and their tendency to interact? How does the model of single atom help you understand what happens when multiple atoms interact to form a compound? Incandescent, fluorescent, and element tube, wavelength, frequency, electromagnetic spectrum, emission, absorption, spectra, spectroscope, nucleus, protons, physical models, conceptual models, quantum, orbit, periodic table Chapter 6 How are cells the fundamental unit of life? How are cells divided through a regulated process? How do membranes control what enters and leaves the cell? How are cells composed of organic molecules? Detailed Questions: How are cells and atoms related? How do you determine total magnification and FOV in a microscope? What is a unique feature you noticed in a plant cell that is meant to be in the light? Why is carbon so important to life? What are the most common elements in living organisms? What are four important basic types of organic molecules and their function? What are the main differences between prokaryotes and eukaryotes? What are some differences/similarities between plant and animal cells? What is osmosis? How is it related to diffusion? What is a concentration gradient? What does selectively permeable mean? What is active transport? Passive transport? What is cell division and mitosis? What is differentiation? Iso/Hypo/hypertonic Organic molecules Carbohydrates Lipids Proteins Enzymes Nucleic acids Macromolecules Field of view Uni/multicellular Organelles Eu/prokaryotes Osmosis Diffusion Concentration gradient Selectively permeable Cell cycle Mitosis Differentiation permeable [parts of the cell] Chapter 7 Essential Questions: How does photosynthesis occur in chloroplasts to convert light energy into chemical energy? How does cellular respiration provide energy for cells to function? Detailed Questions: Where does energy and matter come from in plants? Why do dissolved oxygen levels change in an aquatic system involving plants? How do you compare light as energy for plants to food as energy for animals? What is photosynthesis and what chemical reaction describes it? What is the Calvin Cycle and what does it create? What is ATP and how is it created? What is the source of mass increase in plants during growth? Compare aerobic and anaerobic respiration. What are the stages of cellular respiration? What is the relationship between photosynthesis and cellular respiration? Energy Photosynthesis Chlorophyll ATP Enzymes Calvin cycle Cellular respiration Aerobic Respiration Anaerobic Respiration Krebs cycle Biosynthesis Decomposition Metabolism Catalyze Chapter 10 What role do (or did) constellations serve humans? How do astronomers use stellar parallax to determine the location of stars? How is the parallax created by Earth’s rotation like what we see with our eyes? What variable(s) change the speed of a wave? What does adding energy to a wave do to the wave? How does matter move in a wave? Does a wave transfer matter, energy, or both? How do scientists use the principles of changing EM wave speed through different media to help them understand distant stars? Constellation Parallax Light-year Astronomical Unit Pitch Frequency Amplitude EM Spectrum “c” Chapter 5 Essential Questions: How does energy distinguish chemical from nuclear reactions? How do nuclei fuse together, split apart, or decay? How do elements exist in versions due to the number of neutrons in the nucleus? Detailed Questions: What fuels stars? What are the different recipes for stars? Why do different gases have different densities at the same pressure and temperature? Why could neutrons be called nuclear glue? In what way are new elements made through chemical reactions in stars? Why does the temperature in the center of stars have to be so hot? How is radioactive decay similar and different to nuclear fusion? How can radioactive decay cause the atomic number to increase? Compare fission and fusion? Where did earth get its carbon? What is the evidence for the existence of black holes? Isotopes Nuclear Strong nuclear force Electrostatic force Radioactive decay Half-life Fission Chain reaction Critical mass Neutron Quarks