More Atomic Structure Practice: Use the periodic table to help you
advertisement

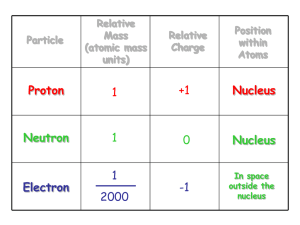
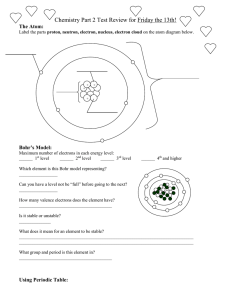
More Atomic Structure Practice: Use the periodic table to help you find the answers! Draw atomic structures and electron dot diagrams for the following atoms: H, He, Li, Be, B, C, O, F, Ne, Na, Al, Si, P, Cl, Ar Here are the atomic structures: NOTE! Only the electrons in their shells are shown below! The number of protons equals the TOTAL number of electrons. The number of neutrons is found below. Here are the electron dot diagrams: How many neutrons are in the following atoms (show your work!): H, He, Li, Be, B, C, O, F, Ne, Na, Al, Si, P, Cl, Ar Atomic mass (rounded) – Atomic Number = # neutrons 0, 2, 4, 5, 6, 6, 8, 10, 10, 12, 14, 14, 16, 18, 22 What are the rows of the periodic table called? periods What are the columns of the periodic table called? groups or families What is the smallest piece of an element? atom What is the smallest piece of a compound? molecule How many naturally occurring elements are there? 92 What is most matter on Earth, elements or compounds? compounds How much smaller is an electron compared to a proton? 2000 times smaller How far are electrons from protons? 10,000 times farther What are valence electrons? outer shell or highest energy electrons What do we call atoms with more or less neutrons in an average atom for any element? isotopes