Applied Density Problems Worksheet
advertisement
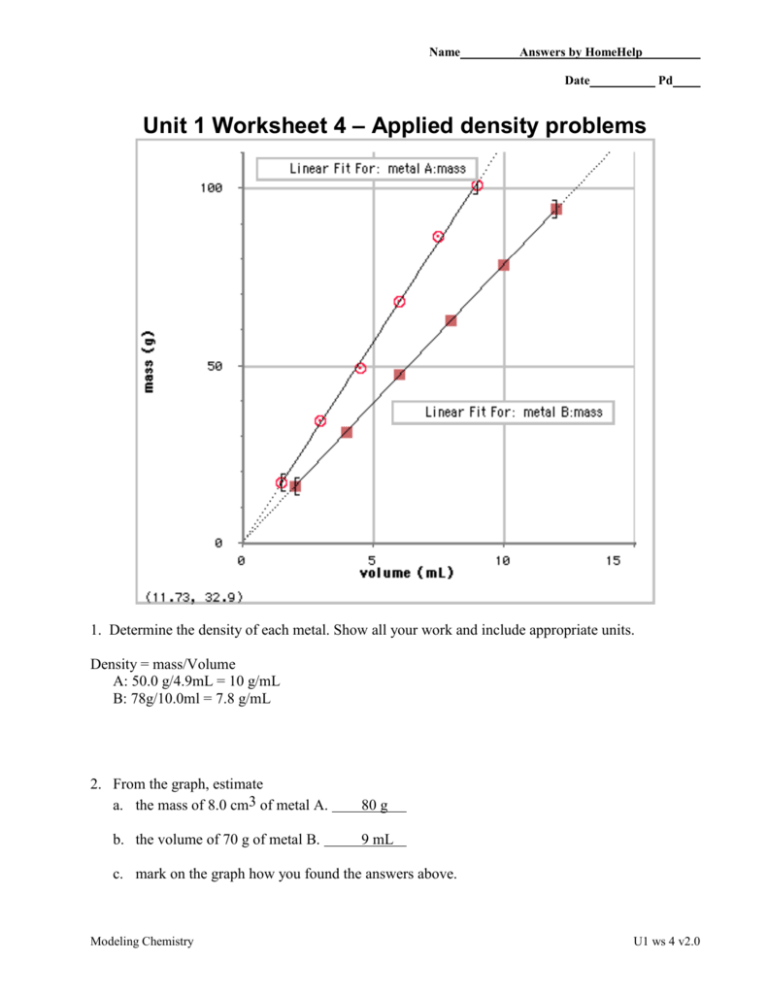
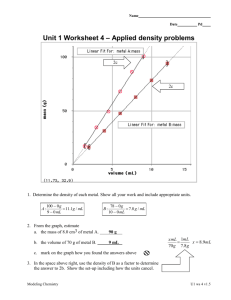
Name Answers by HomeHelp Date Pd Unit 1 Worksheet 4 – Applied density problems 1. Determine the density of each metal. Show all your work and include appropriate units. Density = mass/Volume A: 50.0 g/4.9mL = 10 g/mL B: 78g/10.0ml = 7.8 g/mL 2. From the graph, estimate a. the mass of 8.0 cm3 of metal A. b. the volume of 70 g of metal B. 80 g 9 mL c. mark on the graph how you found the answers above. Modeling Chemistry U1 ws 4 v2.0 3. In the space above right, use the density of B as a factor to determine the answer to 2b. Show the set-up including how the units cancel. V/D=m 70mL/7.8g/mL = Xg 9g = Xg 9g 4. Ethanol has a density of 0.789 g/cm3. a. What is the mass of 225 cm3 of ethanol? 4.5 = V * 100/ 225 V = 10.125 mL mass = 10.125 mL x 0.789 g/mL mass = 7.99 g b. What is the volume of 75.0 g of ethanol? m/D=V 75.0g/0.789g/mL = Xg 95.0g = X 5. What is the density of water in g/mL? What does that mean? 1.00 g/mL. This means that for every mL of water, it will weight 1 gram. 6. The cup is a volume widely used by cooks in the U.S. One cup is equivalent to 237 cm3. If 1 cup of olive oil has a mass of 216 g, what is the density of olive oil in g/cm3? 216 g 0.911g / cm 3 3 237cm 7. What would you expect to happen if the cup of olive oil in question 6 is poured into a container of ethanol? Why? Olive oil is denser than ethanol so it will sink to the bottom of the container. Gold has a density of 19.3 g/ cm3. A cube of gold measures 4.23 cm on each edge: 8. What is the volume of the cube? Volume 30cm x 30cm x 40cm 36,000 cm 3 9. What is its mass? How many significant figures should you include in your answer and why? 19.3 g 1 kg xg x 694,800 g x 694.8 kg, 700 kg (one sig . fig.) 3 1mL 1000 g 36,000 cm Modeling Chemistry U1 ws 4 v2.0 10. A standard backpack is approximately 30cm x 30cm x 40cm. Suppose you find a hoard of pure gold while treasure hunting in the wilderness. How much mass would your backpack hold if you filled it with the gold? An average student has a mass of 70 kg. How do these values compare? The backpack of gold has ten times the mass of an average student. Modeling Chemistry U1 ws 4 v2.0