303 Adipic Acid S072
advertisement

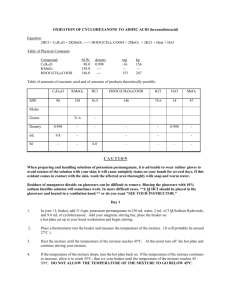
1 OXIDATION OF CYCLOHEXANONE TO ADIPIC ACID O O Mn O OK+ O Potassium Permanganate HO OH O Na+ cyclohexanone O OH- Sodium Hydroxide adipic acid CAUTION When preparing and handling solutions of potassium permanganate, it is advisable to wear rubber gloves to avoid contact of the solution with your skin; it will cause unsightly stains on your hands for several days. If this oxidant comes in contact with the skin. wash the affected area thoroughly with soap and warm water. Residues of manganese dioxide on glassware can be difficult to remove. Rinsing the glassware with 10% sodium bisulfite solution will sometimes work. In more difficult cases, dry abrasive cleanser on the tip of a wet brush will remove the residue. Day 1 1. In your 1 L beaker, add 31.5 gm. (0.2 mol) potassium permanganate in 250 mL water, 1 pipet-full of 3 M Sodium Hydroxide, and 9.8 mL (0.1 mol) of cyclohexanone. Mix these components thoroughly using your longest stirring rod. 2. Place a glass thermometer into the beaker and measure the temperature of the mixture. 3. Heat the mixture on a hat plate ( located at your hood workstation ) until the temperature of the mixture reaches 45oC. IMMEDIATELY, remove the beaker from the hot plate and place it on your bench top and continue stirring your mixture. 4. If the temperature of the mixture drops, return it to the hot plate. If the temperature of the mixture continues to increase, allow it to reach 55oC, then ice your beaker until the temperature of the mixture reaches 45 - 50oC. DO NOT ALLOW THE TEMPERATURE OF THE MIXTURE TO GO BELOW 45oC. 5. When the temperature no longer rises above 50oC upon removal of the beaker from the ice bath, allow the mixture to stand for an additional 5 - 10 minutes; its temperature should drop during this period. 6. Heat the mixture to boiling CAREFULLY, WITH STIRRING on your hot plate (set at 3 - 4) for 5 - 10 minutes, to complete the oxidation. The mixture has been known to bump out of the beaker. 7. While your mixture is heating, attach your 500 mL filter flask assembly to the vacuum source, wet your filter paper with water and turn on the vacuum/water aspirator. 8. Once the mixture in your beaker has begun to boil, test the solution for unchanged permanganate by placing a glass stirring rod into the mixture and removing it. Place that drop on a piece of filter paper. If the spot on the paper is brown with no purple color then all of your permanganate has been reacted. If a purple ring appears around the brown spot then you have to continue boiling the mixture and test it until no purple color appears on the filter paper 2 9. Destroy any excess permanganate by adding small amounts of solid sodium bisulfite to the mixture until the spot test is negative. Do not add a large excess of bisulfite. 10. Filter the mixture by vacuum and rinse the reaction flask and filter cake with two 10-mL portions of hot water. Dispose of the filter cake into the solid recovery container. 11. Rinse out your 1 L beaker and pour the contents of your filter flask back into it. Concentrate the filtrate to about 65 mL by heating on a hot plate, setting 5 - 6. Using a stirring rod in your beaker to prevent bumping while you are concentrating your solution. 12. If the concentrate is colored, refer to your Arene Oxidation experiment and add a small amount of NaHSO3 . If the color persists, add a spatula of decolorizing carbon and re-heat the mixture to boiling for about 2 minutes, then vacuum filter, using your small filter flask and small buchner funnel. 13. Pour the clear, colorless filtrate into a 100 mL beaker and allow it to cool to room temperature in an ice bath. 14. Acidify the filtrate with 20 mL 6M hydrochloric acid and test the solution with blue litmus paper. 15. Cool this mixture in an ice-water bath and isolate the Adipic acid by vacuum filtration, using your 500 mL filter flask and large buchner funnel. 16. Recrystallize your crude product by placing it in a clean 100 mL beaker and adding 20 mL of a 50% Ethanol-water mixture. 17. Bring your solvent product mixture to a boil on your hot plate at your bench. If your solid does not dissolve completely once the mixture starts to boil, add an additional 5 mL of room temperature solvent and again bring the mixture to a boil. Continue this procedure until a final volume of 30 mL is attained. If at this point you still have undissolved material in your beaker, CONSULT YOUR INSTRUCTOR. 18. Ice down an additional aliquot of your 50% Ethanol-water solvent. 19. Remove the boiling mixture from the hot plate and allow it to cool to room temperature on your desk top. 20. At this point, place the beaker containing your product into an ice bath and allow your product to crystallize out of solution. 21. Isolate your product by vacuum filtration. Wash the solid in the funnel with 5 mL of the 50% ethanol-water solvent. 23. Discard your filtrate down the sink. 3 Day 2 Determine the yield, melting point and molecular weight of the pure Adipic acid obtained, and record them in the Results section of your lab report, complete with appropriate calculations. Molecular Weight Determination 1. Write a balanced equation for the titration 2. Weigh approximately 0.1 gm of your dried product into a 250 mL Erlenmeyer Flask. Do your weighing to three decimal places and keep the weight between 0.090 gm and 0.110 gm. Dissolve your sample in 25 mL 95% Ethanol. Titrate the solution with 0.100M NaOH to a faint pink end point, using 3 drops of phenolphthalein as your indicator. Calculate your equivalent weight using the following equation. 3. Calculate your molecular weight molecular weight = 2 x grams of acid ----------------------------------------------------------------( volume of base consumed in liters ) x M 4. Record this calculation in the Results section of your lab report. 5. Be prepared to derive the mathematical equation from the balanced chemical equation. 6. Why are the grams of acid multiplied by 2 ? See Your balanced equation and be able to derive the above from the balanced equation and the titration data. (Hint: It doesn't come from the "2" fairy). Return the remainder of your product, if any, to the jar in the front of the lab. Che S-303 S'07 Lab Report Title: Oxidation of Cyclohexanone to Adipic Acid Name: Date: Equation: 2HCl + C6H10O + 2KMnO4 --> HOOC(CH2)4COOH + 2MnO2 + 2KCl + Heat + H2O Table of Physical Constants Compound M.W. C6H10O 98.0 KMnO4 158.0 HOOC(CH2)4COOH 146.0 density 0.998 --------- mp -16 --153 bp 156 --267 Table of amounts of reactants used and of amounts of products theoretically possible 4 MW Moles C6H10O KMnO4 HCl HOOC(CH2)4COOH KCl H2O MnO2 98 158 36.5 146 74.6 18 87 0.1 0.20 Grams 31.5 Density .998 mL 9.8 Fill in the rest of the boxes IN PENCIL BEFORE you begin the experiment. Show the results to your instructor. Calculate the actual yield, and percent yield (w/w%) for your product Molecualr weight calculation for Adipic Acid: Melting Range for your product: to o C 5 6 Theory behind the Experiment A. Equations: The net equation is the sum of two equations. The first one is a redox equation. The cyclohexanone is oxidized to 1,6-hexanedioic acid (adipic acid) while the permanganate ion is reduced to MnO2. (Which is the oxidizing agent & which is the reducing agent?) C6H10O + 2KMnO4 -----> 2MnO2(s) + C6H8O4K2 + H2O + heat. The second equation describes the reaction which converts dipotassium adipate to adipic acid. Adipic acid is insoluble in water but the dipotassium salt is almost completely soluble in water; therefore the salt is converted to the acid. C6H8O4K2 + 2HCl ----> C6H10O4 + 2KCl. If you add up these two equations, you will obtain the net equation at the beginning of the experiment. B. Procedure: Step 1 OH is needed to start the reaction. It acts as a catalyst. Steps 2-3 The reaction is exothermic but the activation energy is not reached until 45 oC. Above 60oC the reaction tends to run out of control and gas forms so rapidly, that the reaction mixture is violently splattered all over the room. That is why you keep the reaction temperature between 45o + 55oC. Steps 8-9 If permanganate is not destroyed, a pink solid crystallizes out with the hexanedioic acid and contaminates it. Adding an excess of bisulfite can cause some of the manganese dioxide to reduce to manganous ion (Mn+2) which is soluble in water. When the hexanedioic acid crystallizes out, some of it may crystallize out as the manganous salt, rather than the free acid; thus your melting points and melting ranges will indicate very impure substances. Step 10 The brown MnO2 has to be removed from the solution by filtration. The MnO 2 particles are very small and can clog the pores of the filter paper. Step 11 The solution may be unsaturated at this stage, so you want to reduce the volume to where the amount exceeds the solubility limit at 0oC. It was experimentally found that 30 ml would be a convenient volume from which to crystallize the adipic acid. Step 12 If you need charcoal to decolorize the solution, you do Steps 12, 13, and 14. Step 14 You are converting the dipotassium salt of adipic acid (dipotassium adipate) which is soluble in water, into adipic acid or hexanedioic acid which is insoluble in water. Step 15 You are reducing the solubility of adipic acid. Step 17 21 ml of 95% (v/v) ethanol and 9.0 ml of water gives 1:1 or a 50% (v/v) solution of ethanol and water. 21 ml of 95% ethanol gives 19.95 ml ethanol and 1.05 ml of water. The 1.05 ml of water + 19 ml of additional water gives 20.05 ml, which is close enough to 50:50. 7 Step 18 You are dissolving the crystals, thus the molecules of adipic acid become separated from each other enough so that the impurities will float away in the solvent. Step 20 The solvent cools, thus allowing the molecules of adipic acid to come close together while the impurities remain far apart. The molecules begin to attach to each other into crystal formation. The molecules attach to each other to form large enough crystals to be caught by the filter paper but not large enough to trap impurities. 8 EXERCISES: 1. Why is the reaction mixture made alkaline ? 2. Why does acidification of the concentrated reaction mixture cause precipitation of hexanedioic acid ? 3. Why is filter aid used ? 4. Write the balanced equation for the oxidation of cyclohexanone to dipotassium hexanedioate by basic potassium permanganate. Show all work. 5. What transformation must the ketone undergo before it is attacked by permanganate ion ? 6. What is the limiting reagent in this reaction ? Why do you think this reagent rather than the other was made limiting ? 7. Why is it important to destroy all of the potassium permanganate before work-up of the reaction mixture ? 8. Write the reaction that occurs between sodium bisulfite and potassium permanganate in aqueous solution. 9 9. Why does the initially purple solution of potassium permanganate change color as the oxidation progresses ? 10. Why would this oxidative method for the preparation of carboxylic acids be unsatisfactory for the synthesis of pentanoic acid from 4-octanone or 4-nonanone ?