Intel® Teach Program
Essentials Course
Unit Plan Template
Click on any descriptive text, then type your own.
Unit Author
First and Last Name
Team SRT 2
School District
Atlanta Public Schools
School Name
Learning Technology
School City, State
Atlanta, GA
Unit Overview
Unit Title
Systems and Change
Unit Summary
Students will explain the difference between a physical change and a chemical change.
Subject Area
Physical Science
Grade Level
5th
Approximate Time Needed
7 weeks
Unit Foundation
Targeted Content Standards and Benchmarks
S5P1 Students will verify that an object is the sum of its parts.
a. Demonstrate that the mass of an object is equal to the sum of its parts by manipulating and
measuring different objects made of various parts.
S5P2 Students will explain the difference between a physical change and a chemical
change.
a. Investigate physical changes by separating mixtures and manipulating (cutting, tearing,
folding) paper to demonstrate examples of physical change.
b. b. Recognize that the changes in state of water (water vapor/steam, liquid, ice) are due to
temperature differences and are examples of physical change.
c. Investigate the properties of a substance before, during, and after a chemical reaction to
find
National Educational Technology Standards
Technology productivity tools
Students use technology tools to enhance learning, increase productivity, and promote
creativity.
Students use productivity tools to collaborate in constructing technology-enhanced
models, prepare publications, and produce other creative works.
Technology communications tools
Students use telecommunications to collaborate, publish, and interact with peers,
experts, and other audiences.
Students use a variety of media and formats to communicate information and ideas
effectively to multiple audiences.
© 2000-2007 Intel Corporation. All Rights Reserved.
Page 1 of 6
Intel® Teach Program
Essentials Course
Technology research tools
o Students use technology to locate, evaluate, and collect information from a variety of
sources.
Student Objectives/Learning Outcomes
Demonstrate and explain examples of how cutting, tearing, and folding are physical changes;
Separate mixtures; Draw, identify, and explain physical changes
Describe a substance before, during (bubbling, temperature change, gas release), and after a
chemical reaction; Create a chemical reaction
Compare/contrast how the properties of a substance can change physically and chemically
Demonstrate examples of how physical changes occur in water – solid, liquid, and gas
Manipulate and explain the total mass of objects and its parts Weigh and measure various
objects
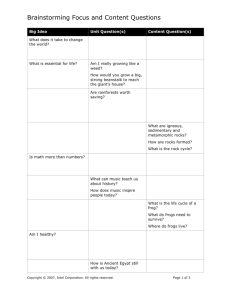
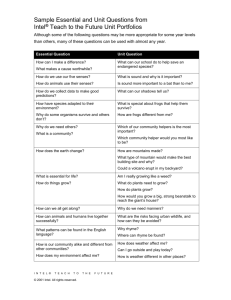
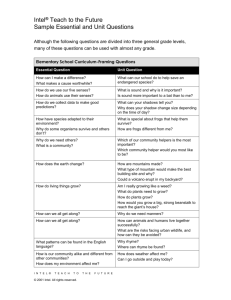
Curriculum-Framing Questions
Essential
Why does matter- matter?
Question
How can matter be changed?
How are mass and weight different?
Why does the mass of an object remain constant regardless of its
location?
Unit
How can I tell if matter has undergone a chemical change or a physical
Questions
change?
How do different forms of water exist on Earth?
How can matter change by just looking at it?
What are some examples of physical changes in matter?
What are some examples of chemical changes in matter?
Content
What is matter?
Questions
What is the law of conservation?
What is mass?
Assessment Plan
Assessment Timeline
© 2000-2007 Intel Corporation. All Rights Reserved.
Page 2 of 6
Intel® Teach Program
Essentials Course
Before project work begins
T-chart /
Venn
Diagram
K-W-L chart
Pretest
Questioning
Sponge
activities
Physical &
Chemical
Changes
journal
Students work on projects
and complete tasks
Observations
Checklist
Rubric
Questioning
Journal
Selfassessment
Research
checklist
After project work is
completed
Cumulating
activity
Post test
Journal
United
Streaming
Brochure
Peer/group
assessment
Conference
Sponge
Assessment Summary
Students will use the journal during the course of the unit. Students may record their
observations using a T-chart or Venn Diagram to compare and contrast the two experiments on
page 1 of the journal. Encourage each group to review and compare their individual
observations.
Complete a K-W-L chart as a class.
Unit Details
Prerequisite Skills
Compare/contrast
Information Literacy/Research skills
Instructional Procedures
Task 1: What’s Happening? Students will pour white powder into a clear liquid. One will have a
very obvious chemical reaction. The other will not. Students will make observations as they
conduct each experiment. Answers will NOT be given. We will use this experiment as a hook for
beginning this unit. Make connections back to this experiment through out the unit. Students will
able to discover what happened in this experiment as they learn about chemical and physical
changes. Students will also create a Physical & Chemical Changes journal they will use during
the course of the unit.
Description: Allow students to work in groups of 2-3. Teacher preps this prior to class. Provide
each group with:
• A bottle 1/3 full of white vinegar and a balloon filled with baking soda stretched over the top,
labeled “A”
• A bottle 1/3 full of water and a balloon filled with baking soda stretched over the top, labeled “B”
© 2000-2007 Intel Corporation. All Rights Reserved.
Page 3 of 6
Intel® Teach Program
Essentials Course
Ask each group to carefully place bottle “A” into the center of the group. Tip the balloon over
allowing the contents of the balloon (baking soda) to spill into the liquid inside the bottle. Ask
students to take notes and record everything they are seeing. Watch carefully looking for any
changes they may see. Repeat the process using bottle “B.” Ensure that students carefully
observe and record all changes they see.
Discussion, Suggestions for use: Create a Physical & Chemical Changes journal to use
during the course of the unit. Students may record their observations using a T-chart or Venn
Diagram to compare and contrast the two experiments on page 1 of the journal. Encourage each
group to review and compare their individual observations.
Next complete a K-W-L chart as a class. Suggestion for K-W-L chart use. Draw a large outline of
a “K,” “W,” and “L” on chart paper. Provide each group with small sticky notes. Have the groups
write the things they know on the sticky notes and post on the “K” and the “W” poster. Save the
“L” for later in the unit. Bring the whole class together to share the information they posted.
Discuss the common observations and questions. As you progress through the unit. Students
will use sticky notes to add comments to the “L” poster.
Possible Solution: When the students combine the ingredients in bottle “A,” they will see
bubbles, gas produced, and balloon will be blown up. White powder (baking soda) will mix and
with the vinegar. Bottle “B” students will see virtually no change. The white powder will clump
and mix with the water. Something was different about the contents of the bottles. Either the
liquids were different or the powers were different. Some students may even recognize that
vinegar and baking soda might have been in the containers.
Allow students to respond in one or two sentences to the Unit EQ: How does matter change?
They may write down questions they have or observations they made related to the EQ.
Task 2: Matter and Its Properties: Changes In Matter Students will view segments of a video
from United Streaming site and complete a variety of vocabulary, hands-on experiments, and
tasks related to the video. Many resources are included with the streaming video.
Description: Visit http://www.unitedstreaming.com and type the title of this task (Matter and Its
Properties: Changes In Matter) into the search bar.
Prior to viewing segments 1 & 2 (Intro. & Physical Changes) distribute the Pre-test from United
Video Streaming. Ask students to complete only numbers 1-4. These questions will serve to
preview to the material students will be learning in the video. Watch the first two segments of the
streaming video. The section is short (4 min and 13 sec). Next, re-watch the video segments
with the Pre-test in hand. Check your answers to the first 4 questions.
Turn to page 2 of the Physical & Chemical Changes journal. Have students answer the following
discussion questions in their journal:
1. What is a physical change?
2. What are some examples of physical changes in matter?
Encourage students to illustrate one or two physical changes they saw in the video segment.
Prior to viewing segment 3 (Chemical Changes) answer questions 5-7 on the pre-test. Put the
pre-test aside and watch video segment 3. Re-watch segment reviewing your answers for
questions 5-7. Turn to page 3 of Physical & Chemical Changes journal. Have students answer
the following discussion questions in their journal:
1. What is a chemical change?
© 2000-2007 Intel Corporation. All Rights Reserved.
Page 4 of 6
Intel® Teach Program
Essentials Course
2. What are some examples of chemical changes in matter?
Encourage students to illustrate one or two chemical changes they saw in the video segment.
Discussion: Have students to share some of the observations they recorded in their journals.
Revisit the K, W, L poster charts. Add new sticky notes to the charts.
Extend learning from school to home. Students will use the data table Changes in Your
Environment provided to record physical and chemical changes they observe at home, around
school, and their neighborhood.
Accommodations for Differentiated Instruction
Resource
Student
Nonnative
English
Speaker
Students who “just don’t get it” will have several accommodations including but not
limited to the following: extra time for study, adjusted learning objectives, modified
assignments, one-on-one assistance from teachers, grouped with a student-expert
on the subject, and/or utilization of manipulatives. Students with disabilities will
have the opportunity to use adaptive technologies and/or answer questions orally
instead of in written format. Students will also have the opportunity to express their
learning or demonstrate an understanding of curriculum by answer fewer questions
Students can also be encouraged to use their first language to process and
reflect on information through journals and discussion.
Pair student with non-English speaking friend
Choose words that students will read, hear, and use frequently.
Select words that provide opportunities for learning other words through
strategies such as looking at word parts, synonyms, homonyms, and antonyms.
Videos and pictures
The lesson can be extended for students who meet and/or exceed standards by
setting up an extension station in the classroom. Students will
Gifted
Student
Content—Provide a variety of ways for students to explore curriculum content
such as independent study and research in the media center
Process--a variety of sense-making activities or processes through which
students can come to understand and "own" information and ideas such as an
independent experiment titled “Observing changes in matter
Product--a variety of options through which students can demonstrate or
exhibit what they have learned by writing a skit that will demonstrate
chemical and physical changes
Materials and Resources Required For Unit
Technology – Hardware (Click boxes of all equipment needed)
Camera
Laser Disk
VCR
Computer(s)
Printer
Video Camera
Digital Camera
Projection System
Video Conferencing Equip.
DVD Player
Scanner
Other
Internet Connection
Television
Technology – Software (Click boxes of all software needed.)
Database/Spreadsheet
Image Processing
Web Page Development
Desktop Publishing
Internet Web Browser
Word Processing
E-mail Software
Multimedia
Other
Encyclopedia on CD-ROM
© 2000-2007 Intel Corporation. All Rights Reserved.
Page 5 of 6
Intel® Teach Program
Essentials Course
Textbook
Georgia Performance Standards (www.georgiastandards.org)
Printed Materials
Lab manuals
Journals
Supplies
Internet Resources
Reference materials
White vinegar
Balloon
baking soda
Water
http://www.bbc.co.uk/schools/ks3bitesize/science/chemistry/chem_react_
intro.shtml
http://www.chem4kids.com/files/matter_chemphys.html
http://chemistry.about.com/od/lecturenotesl3/a/chemphyschanges.htm
http://www.ric.edu/ptiskus/chemical/
http://www.physlink.com/Education/AskExperts/ae244.cfm
Interactive site for students & teachers to become more familiar with
mass and weight:
http://www.edinformatics.com/math_science/mass_volume_density.htm
Good information on the differences between solids, liquids, and gases:
http://www.harcourtschool.com/activity/states_of_matter/
PowerPoint on matter:
http://www.schools.pinellas.k12.fl.us/educators/tec/Davis2/matter.ppt/sld0
01.htm
WebQuest and experiments on Chemical & Physical Changes :
http://mypage.direct.ca/k/kasmith/gina/index.html
sing Oobleck with kids:
http://student.biology.arizona.edu/sciconn/oobleck/oobleck.html
Leaves changing are chemical changes:
http://www.esf.edu/pubprog/brochure/leaves/leaves.htm
On-line Quiz on chemical & physical changes:
http://www.mcwdn.org/chemist/pcchange.html
http://www.quia.com/quiz/303980.html
www.gpb.videostreaming.org
Reference and research:
www.renzulli.com
www.galileo.usg.edu
Virtual Field Trip (Video streaming)
Other Resources
Experiments
Future’s Channel (On-line experiments)
Programs of the Intel® Education Initiative are funded by the Intel Foundation and Intel Corporation.
Copyright © 2007, Intel Corporation. All rights reserved. Intel, the Intel logo, Intel Education Initiative, and Intel Teach Program are
trademarks of Intel Corporation in the U.S. and other countries. *Other names and brands may be claimed as the property of others.
© 2000-2007 Intel Corporation. All Rights Reserved.
Page 6 of 6