File - Mechanical Engineers
advertisement

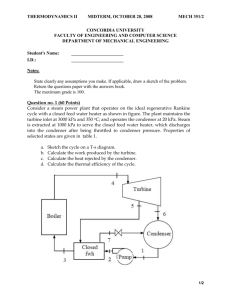
K.L.UNIVERSITY Department of Mechanical Engineering Assignment Test for 2/4 B.Tech. ME - Semester-I -2013-2014 Course Code: Course Title: Course Coordinator: NOTE: 1. 11ME 201 THERMODYNAMICS DR.K.RAMA KRISHNA Date of Exam: Time of Exam: Max. Marks: 16-AUG-2013 9.00AM-9.45AM 10 (i) Answer any TWO question given to you. (ii) Each question carries 5 marks. (iii) Draw neat sketches wherever necessary. (a) The gage pressure of an automobile tire is measured to be200 kPa before a trip and 220 kPa after the trip at a location where the atmospheric pressure is 90 kPa. Assuming the volume of the tire remains constant at 0.035 m 3, determine the percent increase in the absolute temperature of the air in the tire. (Thermodynamics – 6th Edition – Yuns A.Cengel / Michael A Boles - Page No.162, Chapter 3 – Problem -3-116) (b) A spring-loaded piston-cylinder device contains 5 kg of helium as the system as shown in Fig. This system is heated from 100 kPa and 20oC to 800 kPa and 160oC. Determine the heat transferred to and work produced by this system. (Thermodynamics – 6th Edition – Yunus A.Cengel / Michael A Boles - Page No.208, Chapter 4 – Problem -4-72) (c) What is piston displacement work. Derive an expression to determine it in a closed system. 2. (a) A mass of 15 kg of air in a piston-cylinder device is heated from 25 to 77oC by passing current through a resistance heater inside the cylinder. The pressure inside the cylinder is held constant at 300 kPa during the process, and a heat loss of 60 kJ occurs. Determine the electric energy supplied, in kWh. (Thermodynamics – 6th Edition – Yunus A.Cengel / Michael A Boles - Page No.208, Chapter 4 – Problem -4-75) AIR p=constant We Q (b) Air at 90 kPa and -7oC enters an adiabatic diffuser steadily with a velocity of180 m/s and leaves with a low velocity at a pressure of 100 kPa. The exit area of the diffuser is5 times the inlet area. Determine (a) the exit temperature and (b) the exit velocity of the air. (Thermodynamics – 6th Edition – Yunus A.Cengel / Michael A Boles - Page No.261, Chapter 5 – Problem -5-36) P1 = 90 kPa T1 = - 7 0 C AIR V1 = 180 m/s AIR P2 = 100 kPa V2 << V1 A2 = 5A1 (c) Distinguish between intensive and extensive properties. Give few examples for each. 3. (a) The main water line into a tall building has a pressure of 600 kPa at 5 m below ground level, as shown in Fig. A pump brings the pressure up so the water can be delivered at 200 kPa at the top floor 150 m above ground level. Assume a flow rate of 10 kg/s liquid water at 10oC and neglect any difference in kinetic energy and internal energy u. Find the pump work. (Fundamentals of Thermodynamics – Sonntag/ Borgnakke / Van Wylen - Page No.200, Chapter-6 Problem -6.74) Top Floor 150m Ground 5m Water main Pump (b) Two steady flows of air enter a control volume, shown in Fig. One is 0.025 kg/s of flow at 350 kPa, 250oC (state 1), and the other enters at 450 kPA, 15oC (state 2). A single flow exits at100 kPa, -40oC (state 3). The control volume rejects 1kW of heat to the surroundings and produces 4 kW of power output. Neglect kinetic energies and determine the mass flow rate at state 2. (Fundamentals of Thermodynamics – Sonntag/ Borgnakke / Van Wylen - Page No.201, Chapter-6 Problem -6.80) 1 3 2 -Qreject w (c) Distinguish between reversible and irreversible process. 4. (a) The rolling resistance of a car depends on its weight as F=0.006 mg. How far will a car of 1200 kg roll if the gear is put in neutral when it drives at 90 km/h on a level road without air resistance? (Fundamentals of Thermodynamics – Sonntag/ Borgnakke / Van Wylen - Page No.146, Chapter-5 Problem -5.26) (b) A piston/cylinder assembly contains 3 kg of air at 20 oC and 300 kPa. It is now heated in a constant pressure process to 600 K and then expanded adiabatically and returned to its original state by Isothermal compression. (a) Find volume, pressure and temperature at all states (b) Plot the process path in a P- diagram (c) Find the work in the process. (Fundamentals of Thermodynamics – Sonntag/ Borgnakke / Van Wylen - Page No.108, Chapter-4 Problem -4.43) (c) Derive an expression for first law of thermodynamics applied to a steady flow process 5. (a) For analyzing each of the listed events, specify the kind of system you would use and sketch the system boundary, showing any flows of matter. (a) Inflating a bicycle tire with a hand pump (b) Cooling the power supply of a desktop computer (c) The change in size of a toy balloon left in the sun (d) The change in air pressure within a sealed package as temperature changes. (e) Cooling an electric power distribution transformer by spraying water on it. (Engineering Thermodynamics – J.B.Jones / R.E.Dugan - Page No.69, Chapter-1 Problem -1.1) (b) In a quasi equilibrium process in a closed system, a gas expands from a volume of 0.150 m 3 and a pressure of 120kPa to a volume of 0.25m3 in such a manner that p(V+0.030)=constant, where V is in m3. Calculate the work. (Engineering Thermodynamics – J.B.Jones / R.E.Dugan - Page No.74, Chapter-1 Problem -1.75) (c) Define flow energy and explain the term enthalpy. 6. (a) For each of the five cases of processes of a closed system, fill in the blanks where possible: (a) (b) (c) (d) (e) Q 35 kJ 35 kJ 25 kJ W 15 kJ -15 kJ 50 kJ -40 kJ E1 180 kJ E2 ∆E 60 kJ 80 kJ 220 kJ 15 kJ -40 kJ 380 kJ (Engineering Thermodynamics – J.B.Jones / R.E.Dugan - Page No.198, Chapter-3 Problem -3.5) (b) A closed system changes from state A to state B while 55kJ of heat is added and 70 kJ of work is done. As the system is returned to state A, 35 kJ of work is done on it. Determine the heat transfer of process B-A. (Engineering Thermodynamics – J.B.Jones / R.E.Dugan - Page No.198, Chapter-3 Problem -3.6) (c) Define internal energy and prove that it is a point function.