PHR 251 Microbiology I Week 8 practical “Fungi and the Spore Stain
advertisement
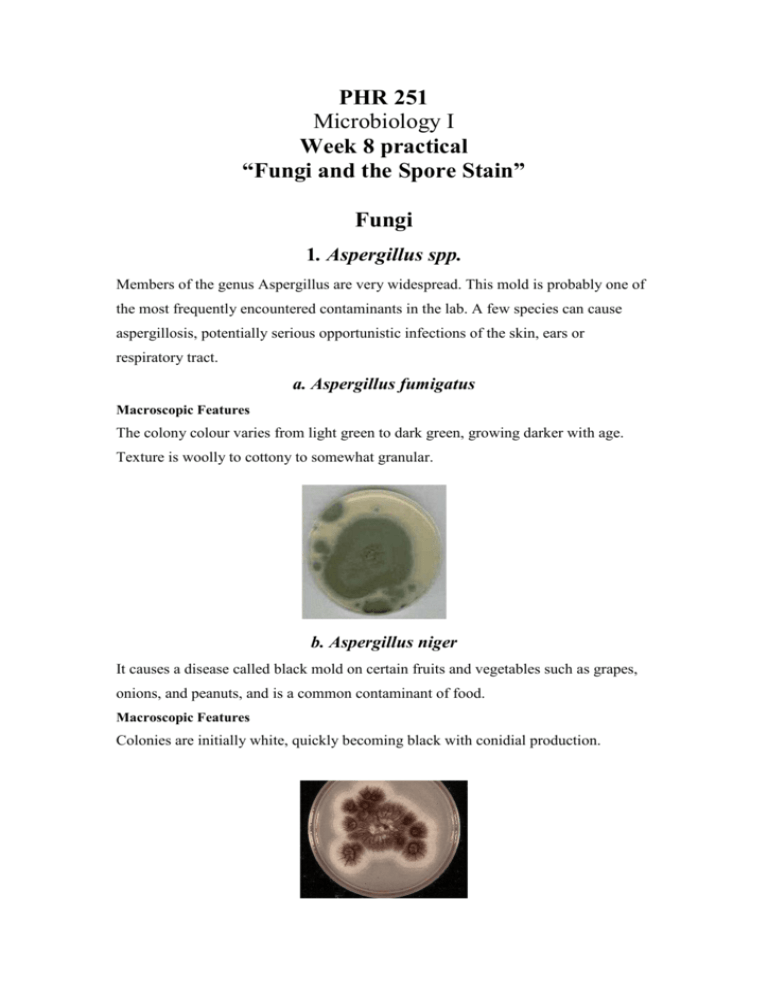
PHR 251 Microbiology I Week 8 practical “Fungi and the Spore Stain” Fungi 1. Aspergillus spp. Members of the genus Aspergillus are very widespread. This mold is probably one of the most frequently encountered contaminants in the lab. A few species can cause aspergillosis, potentially serious opportunistic infections of the skin, ears or respiratory tract. a. Aspergillus fumigatus Macroscopic Features The colony colour varies from light green to dark green, growing darker with age. Texture is woolly to cottony to somewhat granular. b. Aspergillus niger It causes a disease called black mold on certain fruits and vegetables such as grapes, onions, and peanuts, and is a common contaminant of food. Macroscopic Features Colonies are initially white, quickly becoming black with conidial production. 2. Rhizopus spp. Members of the genus Rhizopus are fast growing molds that are common contaminants of food, leading to the spoilage of fruits and vegetables. The common bread mold is one of the Rhizopus species .In immunocompromised patients these molds can cause zygomycosis. Macroscopic Features Colonies of Rhizopus grow very rapidly, fill the Petri dish, and mature in 4 days. The texture is typically cotton-candy like. From the front, the color of the colony is white initially and turns grey to yellowish brown in time. The reverse is white to pale. 3. Candida Members of the genus Candida are the most common cause of opportunistic mycoses. The most significant species is Candida albicans. This yeast colonizes human skin and mucus membranes of the mouth, colon and vagina. Infections caused by this organism are called Candidiasis. Macroscopic Features The colony is usually white, glistening with rhizoid like projections representing pseudo-hyphal formation. Colonies on Sabouraud dextrose agar are white to cream, soft, and smooth to wrinkled. The Spore Stain Spores are highly resistant resting structures produced within cells in a process called sporulation. Sporulation or Spore production is a very important characteristic of some bacteria, allowing them to resist adverse environmental conditions such as desiccation, radiation, chemical exposure, extreme heat and dryness. Because the spores form within the cell they are called endospores. They are resting structures, meaning that there is little or no metabolism. Spores can survive for a very long time, and then under favourable nutrient condition they regerminate and return to the vegetative state (normal cell). The identification of spores is very important for the clinical microbiologist who is analyzing a patient's body fluid or tissue since there are not that many spore-forming genera. In fact, there are two major pathogenic spore-forming genera, Bacillus and Clostridium, together causing a number of lethal diseases---botulism, gangrene, tetanus, and anthrax, to name a few. Principle Spores are impermeable structures which makes them resistant to dying. Spore staining depends on increasing the permeability of the spore coat by heating to permit the dye in. Upon cooling the dye is trapped inside the spore and not allowed out. The primary dye malachite green is a relatively weakly binding dye to the cell wall and spore wall. In fact, if washed well with water, the dye comes right out of the cell wall, however not from the spore wall once the dye is locked in. That is why there does not need to be a decolorizer in this stain. Upon further treatment with Carbol Fuchsin, the Vegetative cell walls will pick up the counterstain carbol fuchsin and will be stained pink. Vegeative Vegetativecell Cell Spore Spore Malachite Malachite Green Green Washing with Washing water with water Carbol Fuchsin Safranin Procedure: 1. 2. 3. 4. 5. 6. Add one drop of saline on a clean slide Aseptically transfer bacteria from the agar plate using a sterilized loop. Spread thoroughly using the loop. Dry and heat fix. Flood the smear with the primary dye, malachite green. Heat the slide till the dye starts to boil then remove the flame. Do not overheat so as not to break the slide. 7. Leave the dye for 5 minutes. Keep the smear moist with the malachite green (always compensate the lost dye). DO NOT let the dye dry. 8. Wash the slide really WELL with water. 9. Flood the smear with the counterstain dye, carbol fuchsin, and leave for 1 minute 10. Wash thoroughly with water 11. Leave to air dry 12. Add one drop of oil and examine under the microscope. PHR 251 Microbiology I Week 8 Practical “Fungi and The Spore Stain” Student Name: UID: Group: Draw a labelled diagram of the stained film: Comment: ………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………… ………………………………………………………………………………………………………………………… Answer The Following Questions: QUESTIONS: 1. What is the purpose of heating in this staining method? 2. Why don’t we need a decolourizer?