trends in plant science
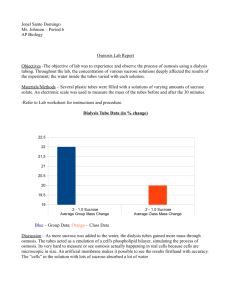
reviews
The sucrose-cleaving enzymes of
plants are crucial for development,
growth and carbon partitioning
Arnd Sturm and Guo-Qing Tang
Sink organs of most plant species are supplied with carbon and energy in the form of
sucrose. The channeling of sucrose into sink metabolism requires its cleavage by several
isoforms of invertase and sucrose synthase, which are localized in different subcellular compartments. These activities regulate the entry of sucrose into distinct biochemical pathways,
such as respiration or biosynthesis of cell wall polysaccharides and storage reserves. Other
vital roles for the sucrose-cleaving enzymes include invertase activity at the site of phloem
unloading and vacuolar invertase and sucrose synthase in sink organs, which drives the
long-distance transport of sucrose. In addition, invertases have been implicated in the
defense response and in turgor-driven cell expansion, and sucrose synthase expression is
associated with low temperature and anaerobiosis responses. Finally, because sugars
also regulate gene expression, the sucrose-cleaving enzymes play a fundamental role in
controlling cell differentiation and development.
T
he different organs of plants have diverse tasks and bio- wall invertase; Fig. 1). Their genes are temporally and spatially
chemical requirements. One of the crucial functions of expressed during plant development, and are regulated by several
source leaves is the synthesis of energy-rich molecules for environmental factors2,3. Vacuolar and cell wall invertases have
the transport of carbon, whereas heterotrophic sink organs, such acidic pH optima and are, therefore, referred to as acid invertases
as developing fruits, seeds, roots and tubers are dependent on the (soluble and insoluble acid invertases, respectively). The amino acid
import and utilization of these compounds. In most plant species, sequences of the plant acid invertases share a few highly conserved
assimilated carbon is transported as sucrose, a disaccharide in motifs and are related to the sequences of invertases from yeast and
which glucose and fructose are linked via an O-glycosidic bond bacteria5,6. By contrast, the sequences of plant neutral or alkaline
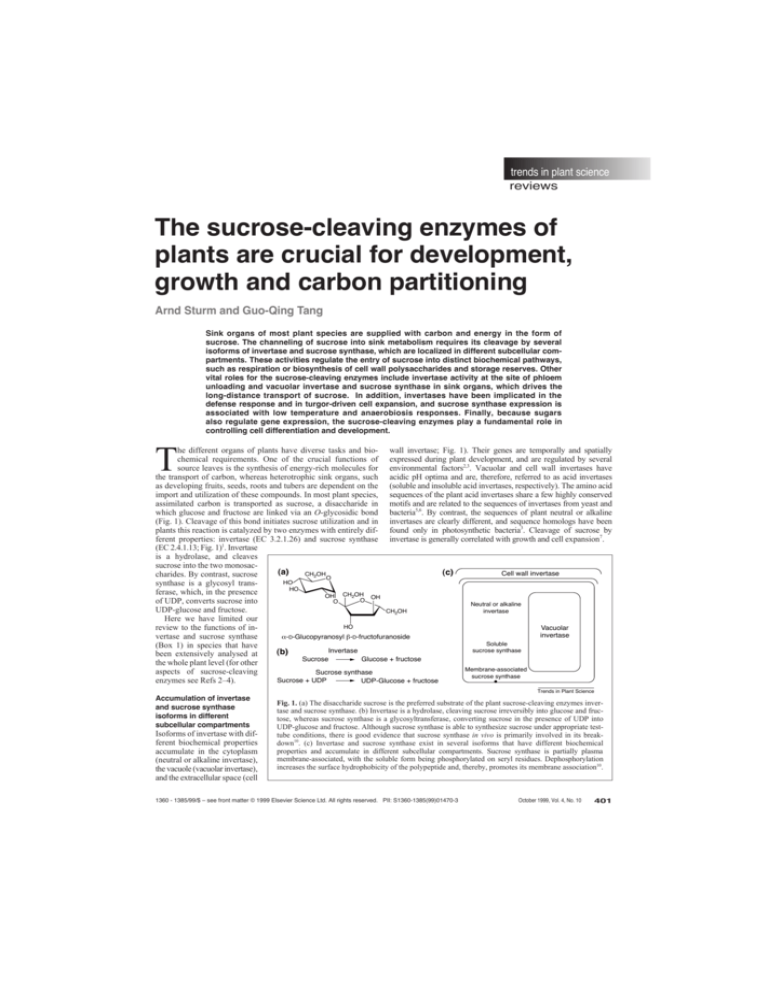
(Fig. 1). Cleavage of this bond initiates sucrose utilization and in invertases are clearly different, and sequence homologs have been
plants this reaction is catalyzed by two enzymes with entirely dif- found only in photosynthetic bacteria3. Cleavage of sucrose by
ferent properties: invertase (EC 3.2.1.26) and sucrose synthase invertase is generally correlated with growth and cell expansion7.
(EC 2.4.1.13; Fig. 1)1. Invertase
is a hydrolase, and cleaves
sucrose into the two monosac(a)
(c)
Cell wall invertase
CH2OH
charides. By contrast, sucrose
O
HO
synthase is a glycosyl transHO
ferase, which, in the presence
OH CH2OH OH
O
O
of UDP, converts sucrose into
Neutral or alkaline
UDP-glucose and fructose.
invertase
CH2OH
Here we have limited our
HO
Vacuolar
review to the functions of ininvertase
vertase and sucrose synthase
a-D-Glucopyranosyl b-D-fructofuranoside
Soluble
(Box 1) in species that have
sucrose synthase
Invertase
(b)
been extensively analysed at
Sucrose
Glucose + fructose
the whole plant level (for other
Membrane-associated
aspects of sucrose-cleaving
Sucrose synthase
sucrose synthase
Sucrose + UDP
UDP-Glucose + fructose
enzymes see Refs 2–4).
Trends in Plant Science
Accumulation of invertase
and sucrose synthase
isoforms in different
subcellular compartments
Isoforms of invertase with different biochemical properties
accumulate in the cytoplasm
(neutral or alkaline invertase),
the vacuole (vacuolar invertase),
and the extracellular space (cell
Fig. 1. (a) The disaccharide sucrose is the preferred substrate of the plant sucrose-cleaving enzymes invertase and sucrose synthase. (b) Invertase is a hydrolase, cleaving sucrose irreversibly into glucose and fructose, whereas sucrose synthase is a glycosyltransferase, converting sucrose in the presence of UDP into
UDP-glucose and fructose. Although sucrose synthase is able to synthesize sucrose under appropriate testtube conditions, there is good evidence that sucrose synthase in vivo is primarily involved in its breakdown10. (c) Invertase and sucrose synthase exist in several isoforms that have different biochemical
properties and accumulate in different subcellular compartments. Sucrose synthase is partially plasma
membrane-associated, with the soluble form being phosphorylated on seryl residues. Dephosphorylation
increases the surface hydrophobicity of the polypeptide and, thereby, promotes its membrane association10.
1360 - 1385/99/$ Ð see front matter © 1999 Elsevier Science Ltd. All rights reserved. PII: S1360-1385(99)01470-3
October 1999, Vol. 4, No. 10
401
trends in plant science
reviews
Box 1. Proposed functions of invertases
and sucrose synthase
Cell wall invertase
• Sucrose partitioning between source and sink organs.
• Response to wounding and infection.
• Control of cell differentiation and plant development.
Vacuolar invertase
• Osmoregulation and cell enlargement.
• Control of sugar composition in fruits and storage organs.
• Response to cold (cold sweetening).
Cytoplasmic invertase
• Largely unknown but is probably involved in channeling
sucrose into metabolism (catabolism).
Sucrose synthase
• Channeling of sucrose into metabolism (anabolism).
• Sucrose partitioning between source and sink organs.
• Response to anaerobiosis and cold.
Most plant species contain at least two isoforms of sucrose
synthase, which usually have highly homologous amino acid
sequences and similar biochemical properties. By contrast, the
regulation of their genes is markedly different and distinct developmental- and organ-specific expression patterns have been
found4,8. The sucrose synthase polypeptides are located in the
cytoplasm and, depending on their phosphorylation status, are soluble or tightly attached to the cellulose synthase complex at the
plasma membrane or the actin cytoskeleton9–11 (Fig. 1). In general,
cleavage of sucrose by sucrose synthase is associated with anabolic processes, in which UDP-glucose is the precursor of numerous compounds8. However, the enzyme has also been localized in
the sieve tube–companion cell complex of source leaves, where it
appears to be involved in the catabolism of sucrose to generate
ATP for phloem loading12.
Expression of yeast invertase in plants
Yeast invertase has been expressed in various organs and different
subcellular compartments of transgenic plants13, such as in the
apoplast of potato leaves14. Because phloem loading in these leaves
involves an apoplastic step, sucrose is cleaved before long-distance
transport. As a consequence, export is blocked and high levels of
soluble sugars and starch accumulate, leading to the repression of
photosynthetic genes. At the same time, the sink organs are
starved of carbon, and the plants produce fewer tubers.
Unloading and transport of sucrose in potato tubers is thought
to occur symplastically. Surprisingly, expression of yeast invertase in the apoplast of transgenic tubers leads to a reduction in
tuber number per plant and an increase in tuber size15. Total tuber
yield per plant on a fresh weight basis increases, whereas production
of tuber biomass (dry weight) remains constant. Expression of the
yeast enzyme in the cytosol of transgenic potato tubers is detrimental to both tuber size and yield. Together, these studies reveal that the
activity of a sucrose-cleaving enzyme at the wrong time and place
has profound effects, drastically altering carbon metabolism and
partitioning, as well as gene expression and plant development.
Possible roles for sucrose cleavage in sink organs
It had been suggested that sucrose transport from source leaves
into sink organs was controlled by ‘sink strength’, the ability of a
sink organ to attract sucrose16. Experiments to unravel the molecular nature of this force have identified the cleavage of sucrose
by cell wall invertase at the site of phloem unloading as one of the
key steps17. By contrast, sucrose synthase appears to be largely
responsible for feeding assimilated carbon into sink metabolism4.
Studies on maize and fava bean (Vicia faba var. minor cv. Fribo)
seeds and carrot tap roots support this model (see below).
Fig. 2. Influence of the expression of antisense mRNA for (a) cell wall invertase, (b) vacuolar invertase and (c) sucrose synthase on the development and habit of transgenic carrot plants. In the roots of the antisense plants shown, enzyme activity is reduced by .90%. The control plant
on the right in each panel is also transgenic but harbors only a promotor fused to the reporter gene23,24.
402
October 1999, Vol. 4, No. 10
trends in plant science
reviews
Maize seeds
In the maize mutant miniature-1, the lack of expression of an
endosperm-specific isozyme of cell wall invertase in only a small
region of the seeds, in the cells of the placentochalazal layer in the
pedicel, causes an early degeneration and withdrawal of maternal
cells from the endosperm18. As a consequence, the transport of
photoassimilates into the developing kernel is interrupted and the
seeds are only a fifth of the normal weight. Thus, invertase-mediated maintenance of a physiological gradient of sucrose between
pedicel and endosperm is crucial for the normal development of
endosperm and seeds.
In maize, two isoforms of sucrose synthase (SH1 and SUS1)
are encoded by the Shrunken1 (Sh1) and Sucrose synthase1 (Sus1)
loci, respectively. Although both genes are expressed in the developing endosperm, SH1 contributes .90% of the total sucrose synthase activity. Seeds of the shrunken1 (sh1) mutant have a mild
starch deficiency and, because of reduced cellulose synthesis, they
also have degenerated storage cells. By contrast, no phenotypic
change is associated with the sus1 mutation, probably because the
huge excess of SH1 protein in the embryos compensates physiologically for the loss of the SUS1 protein. Seeds of the doublerecessive genotype (sh1 sus1) accumulate less starch than sus1
seeds. Detailed biochemical and histochemical analyses of these
seeds demonstrate that the SH1 isozyme preferentially provides
the substrate for cellulose biosynthesis, whereas SUS1 generates
precursors for starch biosynthesis19.
Fava bean seeds
In developing legume seeds, the embryo is isolated from the surrounding seed coat by the apoplast. During the pre-storage phase
of developing fava bean seeds, high levels of hexoses in the
cotyledons and the apoplastic endospermal space correlate with
high levels of cell wall invertase in the seed coat, where invertase
expression is confined to the innermost cell layer20. The hexoses
are taken up by a hexose–proton co-transporter located only in the
epidermal cells of the embryo, and used for growth and cell division rather than storage21. A model has been proposed for an
invertase-mediated unloading process, in which cell wall invertase contributes to establishing sink strength in young seeds20.
At the onset of the storage phase, cell wall invertase activity
declines and, concomitantly, an active sucrose transport system is
formed in the epidermis of the embryo. At this stage, cotyledonary
sucrose metabolism is controlled by a cycle of synthesis and breakdown, involving sucrose-phosphate synthase and sucrose synthase. The net breakdown for storage product synthesis involves
sucrose synthase22.
Carrot tap roots
Different functions of cell wall invertase and sucrose synthase
have also been detected by studies on transgenic carrot plants, in
which the activities of the two enzymes are altered by antisense
expression23. The 35S promoter of CaMV used in these studies is
only active in tap roots and, thus, enzyme activity is exclusively
downregulated in sink organs and not in source leaves. Transgenic
plants expressing antisense mRNA for cell wall invertase develop
no tap roots (Fig. 2), and the small primary-type roots contain
markedly reduced levels of soluble sugars and starch. Because the
biosynthesis of photosynthate continues, the plants develop more
leaves, and these accumulate higher levels of carbohydrates.
Together, these data suggest that carrot cell wall invertase plays a
crucial role in sucrose partitioning to developing tap roots.
In tap roots with reduced sucrose synthase activity, sucrose
utilization is markedly decreased and higher levels of sucrose,
but lower levels of UDP-glucose, glucose, fructose, starch and
cellulose, accumulate24. Both leaves and roots of the antisense
plants are markedly smaller (Fig. 2); although the leaf-to-root dry
weight ratios are unchanged, suggesting that sucrose synthase in
carrot is a major determinant of plant growth, rather than of
sucrose partitioning. In contrast to maize, the product of only one
gene (Sus*Dc1) appears to provide the precursors for both starch
and cellulose biosynthesis in carrot.
It is evident that a turgor pressure gradient, based on sucrose
concentration, is the driving force for assimilate transport from
source leaves into sink organs. The sucrose concentration gradient
depends, among other factors, on the rate of sucrose utilization in
sink tissues. Thus, cleavage of sucrose by all sink-located sucrosecleaving enzymes might contribute to sink strength. The importance of the different enzymes might vary from plant to plant, and
change during plant development.
At well defined developmental stages in some sink organs,
such as the storage phase of fava bean seeds25, sink strength is
principally generated by sucrose synthase activity rather than by
cell wall invertase. Presumably, the conversion of an osmotically
active sugar into an insoluble polymer, such as starch, creates a
strong sucrose sink, making cell wall invertase activity unnecessary.
This hypothesis is further supported by developing potato tubers
and tomato fruits, which have little acid invertase activity but high
sucrose synthase levels. A reduction in sucrose synthase activity
in potato tubers by antisense technology leads to a marked reduction in their starch content and, thereby, to a marked reduction in
tuber yield26. During tomato fruit development, sucrose synthase,
but not acid invertase, is positively correlated with fruit relative
growth rate and with starch content in the pericarp tissue27.
In seeds of maize and fava bean, expression of cell wall invertase is restricted to only a few cells at the transition zone, at the
point where sucrose exits the phloem and enters the storage tissue18,20. Because extracts of developing carrot tap roots do not
contain high levels of transcripts or high levels of cell wall invertase activity28, the expression of the cell wall invertase gene must
also be confined to only a few specialized cells. These cells are
probably in the crown of the storage organ or the cells surrounding vascular bundles (Fig. 3), but have not yet been identified. In
fava beans and cells of Chenopodium rubrum, expression of cell
wall invertase is coupled to the expression of a plasma membranelocated hexose transporter, facilitating the entry of hexoses into
the storage tissue21,29. Thus, in these examples, both cell wall invertase and a sugar transporter appear to contribute to sink strength
and also to control assimilate partitioning by sink-located transfer
and transport processes25 (Fig. 3). After hexose import into the
storage tissue, sucrose appears to be partially resynthesized (Fig. 3).
Sucrose-phosphate synthase activity is frequently found in nonphotosynthetic tissues, and appears to be involved in maize and
fava bean seeds and carrot tap roots. Before sucrose is converted
into storage reserves, it is distributed within the storage tissue by
symplastic transport (or possibly apoplastic transport).
Role of vacuolar invertase
During maturation of tomato fruits, starch deposited during early fruit
development is reconverted into sucrose and, depending on the activity of an acid invertase in the vacuole, is stored mainly as sucrose
or as a mixture of glucose and fructose. Antisense repression of the
fruit vacuolar invertase converts hexose-storing fruit into sucrosestoring fruit without affecting the allocation of assimilated carbon
to the sink organs30. This is rather surprising, and might be
because of the presence of multiple genes for vacuolar invertase.
Post-harvest cold-treatment of potato tubers leads to enhanced
starch degradation and resynthesis of sucrose. Subsequently, a
cold-inducible vacuolar invertase hydrolyses the disaccharide to
October 1999, Vol. 4, No. 10
403
trends in plant science
reviews
Sieve tube Ð
companion cell complex
Invertase
Glu
Suc
Fru
Storage
parenchyma cells
SPS
Transport
UDP-Glu
Suc Storage
Fru-6-P
Metabolism
Trends in Plant Science
Fig. 3. Control of assimilate partitioning by sink-located sugar
transfer and transport processes. Long-distance transport of sucrose
from source leaves into sink organs, such as maize kernels, bean
seeds or carrot tap roots appears to be controlled by sink-located
cell wall invertase and hexose transporters (filled black circle).
In these sink organs, invertase activity appears to be restricted
to only a few specific cells close to where the phloem is unloaded.
For example, the placentochalazal cells of the pedicel of maize
seeds, the transfer cells of the bean seed coat and, possibly, the
companion cells of the phloem of carrot tap roots (as suggested
by this figure). From the apoplast, the hexoses are imported into
the neighboring sink cells, where sucrose is resynthesized by
sucrose-phosphate synthase (SPS) for distribution within the sink
organ. Abbreviations: Suc, sucrose; Glu, glucose; Fru, fructose;
Fru-6-P, fructose-6-phosphate.
glucose and fructose (cold sweetening). Antisense repression of
this cold-inducible invertase in transgenic tubers does not change
the total quantity of soluble sugars that are released in response to
low temperatures. However, it does alter the ratio of hexose to
sucrose in favor of the disaccharide31, confirming the role of vacuolar invertase in the control of sugar composition.
A high correlation between reducing sugar content and petal
expansion in carnation (Dianthus caryophyllus) flowers indicates
that these carbohydrates are the major osmotically active solutes
contributing to growth. The source of these reducing sugars is
thought to be the import and subsequent rapid conversion of
sucrose by a vacuolar invertase32.
Transgenic carrot plants expressing antisense mRNA for the main
form of vacuolar invertase have smaller tap roots (Fig. 2) with
markedly reduced levels of soluble sugars23, suggesting that root
size reduction is at least partially the result of a reduction in cellular
osmotic potential. Thus, not only cell wall-loosening enzymes33
but also osmotic factors, such as hexoses generated by sucrose
cleavage by vacuolar invertases, are crucial for cell expansion. The
results also indicate that in expansion sinks, cleavage of sucrose
by vacuolar invertases can actively drive phloem unloading, and
that their ultimate capacity for storage might increase with each
increment of expansion. This is especially true when vacuolar
invertases provide the primary sucrose cleavage pathway in a cell.
Involvement of sucrose-cleaving enzymes
in stress responses
When plants are subjected to stress, for example because of insect
wounding or pathogen infection, an array of defense reactions is
induced that involves the activation of many genes. Usually, plant
metabolism responds to these stresses by increasing respiration,
by synthesizing wound-sealing compounds, strengthening the cell
404
October 1999, Vol. 4, No. 10
wall, and by producing defense compounds. This increased metabolic activity is probably fed by an enhanced flow of sucrose to
the site of wounding or infection. Evidence for this is the finding
that cell wall and vacuolar invertase activity is rapidly and locally
induced at wound and infection sites5,34, and is accompanied
by the rapid accumulation of high levels of hexose (Fig. 4).
However, the increase in invertase expression could also be
viewed as part of fungal pathogenesis, because fungi use glucose
rather than sucrose.
The hexoses released are not only used as a source of carbon
and energy but they also appear to play an important role in stress
signaling. For example, in tobacco plants overexpressing yeast
invertase in vacuoles or the apoplast, the pathway for systemic
acquired resistance (SAR) is constitutively upregulated35. The
accumulation of pathogenesis-related protein transcripts requires
a certain threshold concentration of hexoses, suggesting that hexose sensing in the secretory pathway is essential for mediating the
activation of defense-related genes (Fig. 4). Because tobacco
plants expressing yeast invertase in the cytosol do not have SAR
responses, the hexose sensor is probably in the endomembrane
system and/or the apoplast (Fig. 4), and it has been hypothesized
that a membrane-associated hexose transporter functions as a
hexose sensor36,37.
Under anaerobic stress, young maize seedlings synthesize
~20 novel proteins (anaerobic proteins), and the synthesis of most
aerobic proteins is halted38. Under these conditions, oxidation of
pyruvate, the end product of glycolysis, cannot occur. To maintain
sufficient energy for cell metabolism, a fermentation pathway is
switched on. As a consequence, the rate of glycolysis increases,
which is achieved in part by the upregulation of sucrose cleavage
by sucrose synthase. In maize, Sh1 and Sus1 respond differently to a
lack of oxygen39. Whereas Sh1 mRNA specifically increases in
response to a prolonged lack of oxygen (anoxia), Sus1 expression
is preferentially and rapidly upregulated by hypoxia (3% O2 in N2)40.
The increase in Sus1 and Sh1 steady-state mRNA is accompanied
by increases in enzyme activity, with SH1 being mainly responsible for total sucrose synthase activity during severe long-term
stress40. In some plant species, such as wheat, expression of the Ss1
gene for sucrose synthase is also induced by low temperatures,
which again helps to meet the increased glycolytic demand41.
Sucrose-cleaving enzymes are crucial for
plant development
Simple sugars, such as sucrose and glucose, are efficient modulators of gene expression42. Numerous sugar-regulated genes have
been identified and the functions of the encoded polypeptides
range from an involvement in plant metabolism to light perception
and cell cycle control36,43 (Fig. 4). In several plant species, the
genes for sucrose synthase and invertase are subjected to sugar
regulation44–47. During development of maize plants, the genes for
sucrose synthase and acid invertase are differentially expressed,
with sugar-enhanced genes (Sus1 and Ivr2) in many importing
organs, whereas sugar-repressed, starvation-tolerant genes (Sh1
and Ivr1) are upregulated primarily during reproductive development. The patterns of expression shift as assimilate partitioning
changes, suggesting integration of the expression of genes that are
differentially responsive to carbohydrate availability (i.e. feast
and famine conditions) and developmental signals45.
During the pre-storage phase of developing fava beans, the
invertase-mediated unloading process leads to a low sucrose-tohexose ratio. At this developmental stage, the cotyledons have a
high meitotic activity and the seed storage functions are impaired.
The transition from the cell division phase to the storage phase is
paralleled by a decrease in invertase activity in the seed coat and a
trends in plant science
reviews
marked increase in the sucrose-to-hexose
ratio and biomass accumulation. Thus,
(a)
embryo growth by cell division correlates
with high hexose levels, whereas cell differentiation and biosynthesis of storage
Sucrose
Sucrose
reserves is triggered by high sucrose conregulation
25
centrations . Proof of the postulated roles
Invertase
Glucose
of sugars in controlling cell fate has been
Glucose
repression
obtained by adding sucrose to mitotically
Alteration of
+
photosynthesis or
active cotyledons in vitro – the cotyledons
Fructose
sugar metabolism
respond by favoring starch accumulation
over cell division. By contrast, a hexosebased medium maintains cell division
rates. It is concluded that invertase controls the carbohydrate status of seed and,
thereby, the fate of seed development
(b)
(invertase control hypothesis for seed
development)25 (Fig. 4).
Marked changes in the activity of acid
Fast
Sucrose
and alkaline invertases appear to be intichange of
Invertase
mately related to the process of cell difsucrose-to-hexose
ferentiation in carrot tissue cultures48.
ratio
Glucose
Upregulation of
Somatic embryogenesis is characterized by
+
defense genes
Fructose
an increase in alkaline invertase and a
and
decrease in acid invertase. Non-embryoinduction of SAR
genic cell lines in contrast with embryogenic cell lines maintain high levels of acid
invertase activity. Assuming that sucrose
participates in plant cell morphogenesis, it
is concluded that the persistence of high
(c)
levels of acid invertase activity at certain
developmental stages could affect differentiation by reducing the level of sucrose25.
Long-term
Sucrose
Thus, the concerted action of two inverchange of
Invertase
tases in the cell might establish the approsucrose-to-hexose
ratio
priate levels of sugars which, by interacting
Glucose
with other components, participate in the
+
Cell proliferation
Fructose
regulation of development (Fig. 4).
versus
Transgenic carrot plants with reduced
cell differentiation
cell wall or vacuolar invertase activities23
Trends in Plant Science
demonstrate phenotypic alterations at early
stages of development. In somatic embryos
Fig. 4. Proposed sugar signaling pathways in plants. Changes in the sugar status of plant cells
expressing antisense mRNA for cell wall
alter the expression of sugar-responsive genes and lead to different physiological responses42.
invertase, the cotyledons fail to separate.
Sugars might regulate plant metabolism (a) via sucrose and glucose-specific sensor molecules, the nature of which remains unclear. Because plant cells appear to measure the influx
By contrast, embryos expressing antisense
of sucrose and glucose into the cytosol, rather than their actual concentrations, it has been
mRNA for vacuolar invertase have cotypostulated that plasma membrane-located sugar transport proteins (filled black circle) in conledons that are two-to-three times as large
junction with hexokinase are involved37. Plant cells are also able to sense rapid changes in the
but have stunted hypocotyls and roots. At
sucrose-to-hexose levels, either in the apoplast or in the endomembrane system. A specific
the stage when control plantlets have two
threshold level of hexose is required before gene expression is induced35. (b) The sensor
to three foliage leaves and one primary
appears to be membrane located (filled black circle), feeding signals toward the expression of
root, shoots of transgenic plantlets expressgenes for defense. (c) Long-term shifts in the sucrose-to-hexose ratio have marked effects on
ing antisense mRNA for either cell wall
cell differentiation and plant development. Cell wall and vacuolar invertase appear to be
invertase or vacuolar invertase are not sepinvolved, but whether the sugar ratio is measured at a membrane or in the cytoplasm is not
arated into individual leaves, but consist of
clear [a hypothetical membrane-located sensor (filled black circle) is shown].
several stunted, interconnected green structures. When transgenic plantlets are grown
on a mixture of sucrose, glucose and fructose rather than sucrose Future perspectives
alone, the malformation is clearly alleviated and plantlets look The sucrose-cleaving enzymes of plants have multiple functions
almost normal. These data further support the hypothesis that that directly or indirectly affect different processes, such as
invertases play a role in plant development (Fig. 4), probably via metabolism, assimilate partitioning, osmoregulation, adaptation
control of sugar composition and metabolic fluxes. This study to cold and low oxygen levels, response to wounding and infecalso extends previous work35 that indicated that invertases can tion, and development (Box 1). Sucrose and its cleavage product
enhance sugar signal transduction regardless of whether they are glucose, appear to be crucial components of the related signal
located in the cell wall or in vacuoles.
transduction pathways (Fig. 4). For example, some genes required
October 1999, Vol. 4, No. 10
405
trends in plant science
reviews
for carbon metabolism are regulated by glucose repression, whereas
others are controlled by sucrose36,37. Thus, plant cells have independent sensors for sucrose and glucose, and sugar receptors
appear to be present in both the plasma membrane and in the cytoplasm. Cells also appear to sense changes in the ratio between
sucrose and glucose and to feed this information into markedly
different signal transduction pathways, such as those leading to
alterations in development or induction of defense reactions. Undoubtedly, the key questions for future study relate to specificity
and coordination.
The molecular nature of the sugar sensors is not known, and
whether a hexokinase is involved remains under dispute49. In
vitro, the activity of acid invertases is inhibited by a small polypeptide of ~17 kDa, termed an invertase inhibitor50,51. Because fairly
low concentrations of sucrose prevent the inhibitor binding to the
enzyme [half-maximum activity at 1.3 mM (Ref. 52)], its physiological function in regulating enzyme activity remains unclear.
However, this unexpected finding suggests that the inhibitor can
sense sucrose and, thus, might be involved in sugar signaling.
The fact that the sucrose-cleaving enzymes have multiple functions has major implications for the manipulation of carbohydrate
metabolism in transgenic plants. In previous studies, unexpected
and surprising pleiotropic effects have been found and, probably,
more will be discovered in the future. However, improved knowledge of these enzymes might allow us to exploit their properties to
specifically alter growth, development, sucrose partitioning or the
pathogen resistance of crop plants.
Acknowledgements
We are grateful to our previous and present collaborators who
contributed to the ideas laid out in this review. We also acknowledge
our colleague Patrick King for critical reading of the manuscript.
References
1 Copeland, L. (1990) Enzymes of sucrose metabolism, Methods Plant
Biochem. 3, 73–85
2 Tymowska-Lalanne, Z. and Kreis, M. (1998) The plant invertases: physiology,
biochemistry and molecular biology, Adv. Bot. Res. 28, 71–117
3 Sturm, A. Invertases: primary structures, functions, and roles in plant
development and sucrose partitioning, Plant Physiol. (in press)
4 Fu, H. and Park, W.D. (1995) Sink and vascular-associated sucrose synthase
functions are encoded by different gene classes in potato, Plant Cell 7,
1369–1385
5 Sturm, A. and Chrispeels, M.J. (1990) cDNA cloning of carrot extracellular
b-fructosidase and its expression in response to wounding and bacterial
infection, Plant Cell 2, 1107–1119
6 Unger, C. et al. (1994) Soluble acid b-fructofuranosidase and comparison with
the cell wall isoenzyme, Plant Physiol. 104, 1351–1357
7 Ricardo, C.P.P. and Ap Rees, T. (1970) Invertase activity during the
development of carrot roots, Phytochemistry 9, 239–247
8 Sturm, A. et al. (1999) Tissue-specific expression of two genes for sucrose
synthase in carrot (Daucus carota L.), Plant Mol. Biol. 39, 349–360
9 Amor, Y. et al. (1995) A membrane-associated form of sucrose synthase and
its potential role in synthesis of cellulose and callose in plants, Proc. Natl.
Acad. Sci. U. S. A. 92, 9353–9357
10 Winter, H., Huber, J.L. and Huber, S.C. (1997) Membrane association of
sucrose synthase: changes during the graviresponse and possible control by
protein phosphorylation, FEBS Lett. 430, 151–155
11 Winter, H., Huber, J.L. and Huber, S.C. (1998) Identification of sucrose
synthase as an actin-binding protein, FEBS Lett. 430, 205–208
12 Lerchl, J. et al. (1995) Impaired photoassimilate partitioning caused by
phloem-specific removal of phyrophosphate can be complemented by a
phloem-specific cytosolic yeast-derived invertase in transgenic plants, Plant
Cell 7, 259–270
406
October 1999, Vol. 4, No. 10
13 Sonnewald, U. et al. (1991) Transgenic tobacco plants expressing yeastderived invertase in either the cytosol, vacuole or apoplast: a powerful tool for
studying sucrose metabolism and sink/source interactions, Plant J. 1, 95–106
14 Heineke, D. et al. (1992) Apoplastic expression of yeast-derived invertase in
potato, Plant Physiol. 100, 301–308
15 Sonnewald, U. et al. (1997) Increased potato tuber size resulting from
apoplastic expression of a yeast invertase, Nat. Biotechnol. 15, 794–797
16 Ho, L.C. (1988) Metabolism and compartmentation of imported sugars in sink
organs in relation to sink strength, Annu. Rev. Plant Physiol. Plant Mol. Biol.
39, 355–378
17 Eschrich, W. (1980) Free space invertase, its possible role in phloem
unloading, Ber. Dtsch. Bot. Ges. 93, 363–378
18 Miller, M.E. and Chourey, P.S. (1992) The maize invertase-deficient
miniature-1 seed mutation is associated with aberrant pedicel and endosperm
development, Plant Cell 4, 297–305
19 Chourey, P.S. et al. (1998) Genetic evidence that two isozymes of sucrose
synthase present in developing maize endosperm are critical, one for cell wall
integrity and the other for starch biosynthesis, Mol. Gen. Genet. 259, 88–96
20 Weber, H. et al. (1995) Seed coat-associated invertases of fava bean control
both unloading and storage functions: cloning of cDNAs and cell type-specific
expression, Plant Cell 7, 1835–1846
21 Weber, H. et al. (1997) A role for sugar transporters during seed development:
molecular characterization of a hexose and a sucrose carrier in fava bean
seeds, Plant Cell 9, 895–908
22 Weber, H. et al. (1996) Sucrose metabolism during cotyledon development of
Vicia faba L. is controlled by the concerted action of both sucrose-phosphate
synthase and sucrose synthase: expression patterns, metabolic regulation and
implications for seed development, Plant J. 9, 841–850
23 Tang, G-Q., Lüscher, M. and Sturm, A. (1999) Antisense repression of
vacuolar and cell wall invertase in transgenic carrot alters early plant
development and sucrose partitioning, Plant Cell 11, 177–189
24 Tang, G-Q. and Sturm, A. Antisense repression of sucrose synthase in carrot
(Daucus carota L.) affects growth rather than sucrose partitioning, Plant Mol.
Biol. (in press)
25 Weber, H., Borisjuk, L. and Wobus, U. (1997) Sugar import and metabolism
during seed development, Trends Plant Sci. 2, 169–174
26 Zrenner, R. et al. (1995) Evidence of the crucial role of sucrose synthase for
sink strength using transgenic potato plants (Solanum tuberosum L.),
Plant J. 7, 97–107
27 Wang, F. et al. (1993) Sucrose synthase, starch accumulation, and tomato fruit
sink strength, Plant Physiol. 101, 321–327
28 Sturm, A. et al. (1995) Development- and organ-specific expression of the
genes for sucrose synthase and three isoenzymes of acid b-fructofuranosidase
in carrot, Planta 195, 601–610
29 Ehneß, R. and Roitsch, T. (1997) Co-ordinated induction of mRNAs for
extracellular invertase and a glucose transporter in Chenopodium rubrum by
cytokinins, Plant J. 11, 539–548
30 Klann, E.M., Hall, B. and Bennett, A.B. (1996) Antisense acid invertase
(TIV1) gene alters soluble sugar composition and size in transgenic tomato
fruit, Plant Physiol. 112, 1321–1330
31 Zrenner, R., Schüler, K. and Sonnewald, U. (1996) Soluble acid invertase
determines the hexose-to-sucrose ratio in cold-stored potato tubers, Planta
198, 246–252
32 Woodson, W.R. and Wang, H. (1987) Invertases of carnation petals. Partial
purification, characterization and changes in activity during petal growth,
Physiol. Plant. 71, 224–228
33 Cosgrove, D.J. (1997) Relaxation in a high-stress environment: the molecular
bases of extensible cell walls and cell enlargement, Plant Cell 9, 1031–1041
34 Benhamou, N., Genier, J. and Chrispeels, M.J. (1991) Accumulation of
b-fructosidase in the cell walls of tomato roots following infection by a
fungal wilt pathogen, Plant Physiol. 97, 739–750
35 Herbers, K. et al. (1996) Systemic acquired resistance mediated by the ectopic
expression of invertase: possible hexose sensing in the secretory pathway,
Plant Cell 8, 793–803
trends in plant science
reviews
36 Jang, J-C. and Sheen, J. (1997) Sugar sensing in higher plants, Trends Plant
Sci. 2, 208–214
37 Smeekens, J. and Rook, F. (1997) Sugar sensing and sugar-mediated signal
transduction in plants, Plant Physiol. 115, 7–13
38 Rowland, L.J., Chen, Y-C. and Chourey, P.S. (1989) Anaerobic treatment
alters the cell-specific expression of Adh-1, Sh, and Sus genes in roots of
maize seedlings, Mol. Gen. Genet. 218, 33–40
39 Taliercio, E.W. and Chourey, P.S. (1989) Post-transcriptional control of
sucrose synthase expression in anaerobic seedlings of maize, Plant Physiol.
90, 1359–1364
40 Zeng, Y. et al. (1998) Differential regulation of sugar-sensitive sucrose
synthases by hypoxia and anoxia indicate complementary transcriptional and
posttranscriptional responses, Plant Physiol. 116, 1573–1583
41 Maraña, C., García-Olmedo, F. and Carbonero, P. (1990) Differential
expression of two types of sucrose-synthase-coding genes in wheat in response
to anaerobiosis, cold shock and light, Gene 88, 167–172
42 Koch, K.E. (1996) Carbohydrate-modulated gene expression in plants, Annu.
Rev. Plant Physiol. Plant Mol. Biol. 47, 509–540
43 Lalonde, S. et al. (1999) The dual function of sugar carriers: transport and
sugar sensing, Plant Cell 11, 707–726
44 Koch, K.E. et al. (1992) Sugar levels modulate differential expression of
maize sucrose synthase genes, Plant Cell 4, 59–69
45 Xu, J. et al. (1996) A similar dichotomy of sugar modulation and
developmental expression affects both paths of sucrose metabolism:
evidence from a maize invertase gene family, Plant Cell 8,
1209–1220
46 Roitsch, T., Bittner, M. and Godt, D.E. (1995) Induction of apoplastic
invertase of Chenopodium rubrum by D-glucose and a glucose analog and
tissue-specific expression suggests a role in sink–source regulation, Plant
Physiol. 108, 285–294
47 Ehness, R. and Roitsch, T. (1997) Differential effect of D-glucose on the level
of mRNAs for three invertase isoenzymes of Chenopodium rubrum, J. Plant
Physiol. 150, 514–519
48 Silva, M.P. and Ricardo, C.P.P. (1992) b-Fructosidases and in vitro
dedifferentiation–redifferentiation of carrot cells, Phytochemistry 31, 1507–1511
49 Halford, N.G., Purcell, P.C. and Hardie D.G. (1999) Is hexokinase really a
sugar sensor in plants? Trends Plant Sci. 4, 117–120
50 Krausgrill, S. et al. (1998) In transformed tobacco cells the apoplastic
invertase operates as a regulatory switch of cell wall invertase, Plant J. 13,
275–280
51 Greiner, S., Krausgrill, S. and Rausch, T. (1998) Cloning of a tobacco
apoplastic invertase inhibitor, Plant Physiol. 116, 733–742
52 Weil, M. et al. (1994) A 17-kDa Nicotiana tabacum cell-wall peptide acts as
an in vitro inhibitor of the cell-wall isoform of acid invertase, Planta 193,
438–445
Arnd Sturm* and Guo-Qing Tang are at Friedrich Miescher
Institute, Maulbeerstrasse 66, CH-4058 Basel, Switzerland.
*Author for correspondence (tel 141 61 697 7625;
fax 141 61 697 3976; e-mail sturm@fmi.ch).
Root gravitropism: a complex
response to a simple stimulus?
Elizabeth Rosen, Rujin Chen and Patrick H. Masson
Roots avoid depleting their immediate environment of essential nutrients by continuous
growth. Root growth is directed by environmental cues, including gravity. Gravity sensing
occurs mainly in the columella cells of the root cap. Upon reorientation within the gravity
field, the root-cap amyloplasts sediment, generating a physiological signal that promotes the
development of a curvature at the root elongation zones. Recent molecular genetic studies in
Arabidopsis have allowed the identification of genes that play important roles in root gravitropism. Among them, the ARG1 gene encodes a DnaJ-like protein involved in gravity signal
transduction, whereas the AUX1 and AGR1 genes encode proteins involved in polar auxin
transport. These studies have important implications for understanding the intra- and intercellular signaling processes that underlie root gravitropism.
P
lants use the information derived from multiple environmental parameters to direct the growth of their organs. This
process allows them to seek out light and nutrients in a
resource-limited environment. A germinating seedling provides a
good illustration of this ability. A seedling quickly has to direct its
shoot to grow upwards towards light, where it can photosynthesize, and to direct its root to elongate downwards into the soil, to
anchor the plant and take up the water and mineral ions needed for
growth and development (Fig. 1). Both organs use gravity and
light, among other stimuli, as directional cues for growth. The
downward growth response to gravity is termed positive gravitropism. Any deviation of the root tip away from its default
growth vector activates a complex signal-transduction pathway
that results in the production of a gravitropic curvature at the elongation zones (Fig. 1).
Where is gravity sensed within the roots?
In roots, centrifugation and surgical- or laser-ablation experiments have indicated that gravity sensing primarily occurs in the
root cap1,2 (Fig. 2). The cap covers the root apical meristem and
protects it from mechanical damage. The cap also produces a variety of compounds that facilitate root growth in soil, and allows the
growth of beneficial microorganisms to form a biological rhizosphere. The cap is composed of central columella and lateral rootcap cells that are continuously being replaced by the division of
specific initials at the root apical meristem3.
1360 - 1385/99/$ Ð see front matter © 1999 Elsevier Science Ltd. All rights reserved. PII: S1360-1385(99)01472-7
October 1999, Vol. 4, No. 10
407