120019332_E-EPCS_R1_BATCH8_100603
1
4
2
3
Sucrose
S
Ed Etxeberria
University of Florida, Lake Alfred, Florida, U.S.A.
5
6
INTRODUCTION
7
8
9
10
11
12
13
14
15
16
17
18
19
20
21
22
23
24
25
26
27
Sucrose plays a unique role in the plant kingdom. Aside
from being the primary product of photosynthesis and the
main form of carbon transport in plants, sucrose constitutes the most abundant form of soluble storage carbohydrate and also serves as a signaling molecule that triggers
essential metabolic events. Furthermore, sucrose plays a
key role in plant reproduction and propagation. In nectars,
sucrose concentration can determine the type and fre- 52
quency of visiting pollinators, which may change with the 53
sexual state of the flower, and its presence in fruits serves
as an attractant to animals for seed dispersal. A readily 54
available source of energy, sucrose sustains the initial 55
stages of growth after dormant periods in temperate plants. 56
From photosynthetic cells in leaves to heterotrophic root 57
cells, sucrose is found in virtually every living plant cell. 58
There are other soluble saccharides present in plants, and 59
in all cases they are accompanied by high levels of 60
sucrose. In effect, sucrose is the ultimate building block 61
for all other organic compounds in plants and most other 62
carbohydrates in nature, given the position of plants as the 63
64
cornerstone of the energy food chain.
28
WHY SUCROSE?
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
The reason for the ubiquitous position of sucrose in the
plant kingdom is not evident. In comparison with trehalose, the other prominent disaccharide found in nature
with equivalent functions in insects and fungi, and with
raffinose-based saccharides, which are commonly found
in various plant species, several conjectures have been
made.[2] Based on the process of natural selection to
perform equivalent functions, the general premise is that
these molecules must share important properties that impart physiological advantages. A common characteristic
to all aforementioned saccharides is their nonreducing
nature. Nonreducing molecules are less reactive and less
susceptible to breakdown by the cellular enzymatic milieu. Their high energy of hydrolysis conserved in their
glycosidic linkage makes these molecules more valuable
as energy currency and as a readily available carbon
Encyclopedia of Plant and Crop Science
DOI: 10.1081/E-EPCS 120019332
Copyright D 2004 by Marcel Dekker, Inc. All rights reserved.
46
47
48
49
50
51
65
66
67
68
69
70
71
72
73
74
75
76
77
78
79
80
81
82
83
84
85
source. Two other disaccharides found in living systems,
maltose and lactose, have glycosidic linkages with less
than half the energy of hydrolysis of sucrose. Finally, both
of these molecules have been shown to protect membrane
lipids during dehydration and freezing, and to help stabilize organelles and proteins.
FROM PHOTOSYNTHETIC PRODUCT
TO THE PHLOEM STREAM
In green cells, glucose 1-phosphate and fructose 6-phosphate are synthesized in the cytosol from triose-phosphates that are produced in the Calvin cycle and exported
from the chloroplast. Sucrose is synthesized from UDPglucose and fructose-6-phosphate in a sequence of two
reactions catalyzed by sucrose-phosphate synthase (SPS)
and sucrose-phosphate phosphatase (SPP). Both enzymes
are localized in the cytosol and appear to form a metabolic
unit during synthesis.[3] Regulation of sucrose synthesis is
a complex system that involves fine and coarse control.[4]
Although some controlling elements have been described,
it is likely that other factors yet to be discovered may
contribute to the overall regulation and may modify
prevailing opinions about sucrose synthesis. In photosynthetic cells, newly synthesized sucrose has two potential
fates depending on cellular, physiological, and environmental factors. Sucrose is either stored in the vacuole and/
or exported to supply carbon to heterotrophic cells. Temporary storage of excess sucrose in the vacuole usually
takes place at times of high photosynthetic activity and
limited phloem loading capacity. The mechanisms of
sucrose transport into the vacuole of green cells are not
fully understood, but the process is believed to be mediated through a passive transport mechanism.[5]
The movement of sucrose from mesophyll cells to the
phloem elements can take various routes depending on the
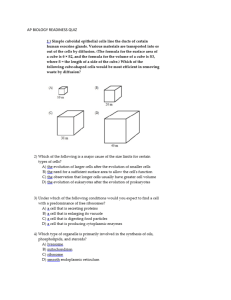
plant species, and it involves different cell types (Fig. 1).
One route consists of intracellular symplastic movement
of sucrose across the plasmodesmata of leaf cells and
eventual release into the sieve element/companion cell
(SE/CC). In the second route, sucrose is released into the
extracellular apoplast at some point, where it diffuses
1
AQ1
F1
120019332_E-EPCS_R1_BATCH8_100603
2
Sucrose
Fig. 1 The cycle of sucrose in a plant, from its synthesis to its storage and utilization. Synthesized in the leaves from photosynthetic
products, sucrose is exported to support heterotrophic cells and/or stored temporarily. Along the transport route, the involvement
of several carriers is required, either for retrieval of leaked sucrose or as part of the apoplastic route. Once stored, the direction of
sucrose transport is reversed to sustain developing plant parts. Loss of sucrose to herbivore consumption can occur at many points along
the route. (Go to www.dekker.com to view this figure in color.)
86
87
88
89
90
91
through the cell wall milieu. After reaching the SE/CC 103
complex, sucrose is retrieved by the well characterized 104
plasmalemma-bound sucrose/H+ symport.[6] Accumula- 105
tion of sucrose in the SE/CC increases the hydrostatic 106
pressure, which drives mass flow transport to other plant 107
parts via the phloem.
108
92
93
FROM THE PHLOEM TO
HETEROTROPHIC CELLS
94
95
96
97
98
99
100
101
102
It is not known whether release of sucrose along the
phloem pathway or at the sink end of the phloem route is
also proton coupled, occurs by diffusion, or involves transport through the symplast along heterotrophic cells. Sieve
element unloading invariably includes an apoplastic component, but its contribution to the overall unloading process depends on many factors and seems to be restricted
to specialized circumstances and tissues. Symplastic unloading and transport apparently constitute the principal
109
110
111
112
113
114
115
116
117
118
119
120
121
122
123
unloading route. In most cases, there is evidence indicating
that sucrose exits the phloem cells and is transported to
heterotrophic cells through plasmodesmata connections.
Although plasmodesmata connections are present along the
entire length of the transport path, efflux seems to occur
only at specific regions (Fig. 1).[6]
That sucrose and other photoassimilates are transported into heterotrophic cells through the symplast has
been largely inferred from the existence of plasmodesmata connections, the observed transport of large protein
or fluorescent probes to the storage cells, and the use of
transport inhibitors and transgenic plants. However the
presence of plasmodesmata is not a universal characteristic within heterotrophic cells, and transport through the
apoplast is undoubtedly required in some instances.[7] The
apoplastic route is necessary in cases where there is symplastic discontinuity between two tissues, as is the case for
filial and maternal tissue in developing seeds. Once released into the apoplast, sucrose may be hydrolyzed into
glucose and fructose by cell wall-bound invertases to
maintain a concentration gradient. The situation is quite
120019332_E-EPCS_R1_BATCH8_100603
Sucrose
3
124 complicated, given that in some organs (such as potato) 169 fore, the mechanisms of sucrose export depend on the
125 both types of postphloem transport take place, depending 170 ultimate fate of the disaccharide. For internal metabolic
126 on developmental stage.
171 use, sucrose can be exported from the vacuole to the
172 cytosol by an ATP-dependent sucrose pump.[8] The ex173 ported sucrose can be either released as a disaccharide or
174 catabolized after export by sucrose synthase, which forms
175 a metabolic unit with the ATP-dependent sucrose pump to
127 STORAGE OF EXCESS SUCROSE
176 release UDP-glucose and fructose.
For external transport, a reverse vesicle-mediated sys128 Whereas some of the sucrose entering the cell is utilized to 177
129 satisfy immediate metabolic demands, excess sucrose is 178 tem (exocytosis) carries sucrose and other vacuolar sub[9]
130 stored in the vacuole for future needs. The mechanism of 179 stances to the apoplast (Fig. 1). Once released into the
131 sucrose transport into the vacuole of heterotrophic cells 180 apoplast, sucrose is transported to growing points through132 depends on its entrance pathway into the cell. Symplas- 181 out the plant to maintain growth, and in a large number of
133 tically loaded sucrose needs to traverse only one mem- 182 biennial plants, to sustain the entire second year repro134 brane barrier into the vacuole: the tonoplast, which is 183 ductive activities. In many ways, sucrose secretion as part
135 believed to possess a sucrose/H+ antiport. Such an antiport 184 of flower nectar follows a similar exocytotic route. How136 system has been identified at the tonoplast of a few 185 ever, it is believed that some solutes in the nectar originate
137 storage cells such as red beet (Beta vulgaris) and Japanese 186 in other cellular organelles and that a more complex
[9]
138 artichoke (Stachys sieboldii), but this system is conspic- 187 network of vesicle transport is involved.
139 uously absent from the high-sucrose-storing cells of
140 sugarcane (Saccharum officinarum) and sweet lime (Ci141 trus limettioides).
142
Where sucrose unloading takes the apoplastic route, 188 COMPLETING THE CYCLE
143 the plasmalemma offers an additional barrier to accu144 mulation. A sucrose symporter similar to that located at 189 Whether originating from the storage organs or from
145 the plasmalemma of SE/CC is presumed to carry sucrose 190 neighboring exporting photosynthetic leaves, sucrose
146 into the cytosol. However, a plasmalemma-bound sucrose 191 provides the energy for the development of new leaves
147 symport in storage cells has been inferred from gene 192 until they become fully autotrophic. A series of soluble
148 expression studies, but its activity has never been 193 and wall-bound invertases, in addition to sucrose syn149 demonstrated directly. More recently, an endocytotic 194 thase, channel sucrose to different metabolic pathways.
150 system of transport has been proposed to carry sucrose 195 Once the leaf becomes an autotrophic organ, the direction
151 (and other dissolved solutes) from the apoplast to the 196 of sucrose flow reverses and export of sucrose renews the
152 vacuole of storage cells. Endocytotic vesicles would 197 cycle. Therefore, as the primary product of photosynthe153 transport solutes to be stored directly into the vacuole, 198 sis, sucrose powers life on earth by virtue of being the
154 whereas the plasmalemma-bound sucrose symporter al- 199 basic fuel for life.
155 lows the passage of sucrose required by cytosolic acti156 vities. In this way, the cytosolic homeostasis is not
157 disrupted by the constant fluctuations of the phloem
158 contents (Fig. 1).
200 ACKNOWLEDGMENTS
159 UTILIZATION AND MOBILIZATION
160 OF RESERVE SUCROSE
161
162
163
164
165
166
167
168
201 This research was supported by the Florida Agricultural
202 Experiment Station, and approved for publication as
203 Journal Series No._____________.
Metabolic demands for long-term stored vacuolar sucrose
occur in vital processes such as resumption of growth in 204
dormant or reproductive tissues, seed germination, and the
maintenance of cell viability in stored commodities.[8] 205
Depending on metabolic demand, stored sucrose can be 206
mobilized by storage cells to supply their own physio- 207
logical requirements and those of remote cells, such as 208
developing shoots, roots, and reproductive organs. There- 209
ARTICLES OF FURTHER INTEREST
Modulation of Gene Expression in Plants by Sugars in
Response to Changes in the Environment, p. XXX
Photosynthate Partitioning and Transport, p. XXX
Plant Response to Stress: Source-sink Regulation by
stress, p. XXX
S
AQ2
120019332_E-EPCS_R1_BATCH8_100603
4
210 REFERENCES
AQ3 211 1. Smeekens, S.; Rook, F. Sugar induced signal transduction in
212
plants. Annu. Rev. Plant Physiol. Mol. Biol. 2000, 51, 49 –
213
81.
214 2. Pontis, H.G. The Riddle of Sucrose. In Plant Biochemistry;
215
Northcote, D.H., Ed.; University Park Press: Baltimore,
216
MD, 1977.
217 3. Echeverria, E.; Salvucci, M.E.; Gonzalez, P.C.; Paris, G.;
218
Salerno, G.L. Physical and kinetic evidence for an as219
sociation between sucrose-phosphate synthase and sucrose220
phosphate phosphatase. Plant Physiol. 1997, 115, 223 –
221
227.
222 4. Huber, S.C.; Huber, J.L. Role and regulation of sucrose223
phosphate synthase in higher plants. Annu. Rev. Plant
224
Physiol. Mol. Biol. 1996, 47, 431 – 444.
Sucrose
225
226
227
228
229
230
231
232
233
234
235
236
237
238
239
240
5.
6.
7.
8.
9.
Kaiser, G.; Heber, U. Sucrose transport into vacuoles isolated from barley mesophyll protoplasts. Planta 1984, 161,
562 – 568.
Lalonde, S.; Boles, E.; Hellman, H.; Barker, L.; Pattrick,
J.W.; Frommer, W.B.; Ward, J.M. The dual function of
sugar carriers: Transport and sugar sensing. Plant Cell 1999,
11, 707 – 726.
Patrick, J.W. Phloem unloading: Sieve element unloading
and post-sieve element transport. Annu. Rev. Plant Physiol.
Mol. Biol. 1997, 48, 191 – 222.
Echeverria, E.; Gonzalez, P.C. ATP-induced sucrose efflux from red-beet tonoplast vesicles. Planta 2000, 211, 77 –
84.
Echeverria, E. Vesicle mediated solute transport between
the vacuole and the plasma membrane. Plant Physiol. 2000,
123, 1217 – 1226.