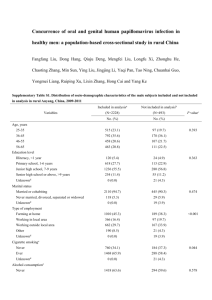
Interaction of viral oncoproteins with cellular target molecules
advertisement

2010 The Authors Journal Compilation 2010 APMIS DOI 10.1111/j.1600-0463.2010.02618.x APMIS 118: 471–493 Interaction of viral oncoproteins with cellular target molecules: infection with high-risk vs low-risk human papillomaviruses DAVID PIM and LAWRENCE BANKS International Centre for Genetic Engineering and Biotechnology (ICGEB), Trieste, Italy Pim D, Banks L. Interaction of viral oncoproteins with cellular target molecules: infection with highrisk vs low-risk human papillomaviruses. APMIS 2010; 118: 471–493. Persistent infection by a subgroup of so-called high-risk human papillomaviruses (HPVs) that have a tropism for mucosal epithelia has been defined as the cause of more than 98% of cervical carcinomas as well as a high proportion of other cancers of the anogenital region. Infection of squamous epithelial tissues in the head and neck region by these same high-risk HPVs is also associated with a subset of cancers. Despite the general conservation of genetic structure amongst all HPV types, infection by the low-risk types, whether in genital or head and neck sites, carries a negligible risk of malignant progression, and infections have a markedly different pathology. In this review, we will examine and discuss the interactions that the principal viral oncoproteins of the high-risk mucosotrophic HPVs and their counterparts from the low-risk group make with cellular target proteins, with a view to explaining the differences in their respective pathology. Key words: Human papillomaviruses; E6; E7; pRB; p53; PDZ proteins; chromosomal instability. David Pim, International Centre for Genetic Engineering and Biotechnology (ICGEB), Padriciano 99, 34012 Trieste, Italy. e-mail: pim@icgeb.org More than 25 years of research into the life cycle and transforming properties of human papillomavirus (HPV) types that infect mucosal epithelia has established a direct causal relationship between viral infection by a subset of these viruses, termed ‘high-risk’ and carcinoma of the uterine cervix (1, 2). Although infection by ‘lowrisk’ HPV types is associated with a negligible risk of malignant progression in laryngeal and tracheal mucosa, HPV-6 and -11 are responsible for recurrent respiratory papillomatosis (RRP), a rare but life-threatening condition that can convert to cancer (3, for review). HPV-6 and -11 infection in genital mucosa causes genital warts (condyloma acuminata) whose uncontrolled growth in rare instances can lead to a condition Invited review known as Buschke-Lowenstein giant condyloma; both of these conditions, although not life-threatening, nevertheless, impose a significant financial burden on health systems worldwide (4). The viral types associated with cervical cancer also play a causal role in a subset of other cancers of the anogenital region, and can infect mucosal tissues of the head and neck region where they are associated with a subset of squamous cell carcinomas (SCCs). Previous studies correlate the risk of cervical cancer with persistent infection by these high-risk papillomavirus types, and at the molecular level, the interaction between the major viral oncoproteins and cellular proteins provides a functional model for the initiation and progression of malignant disease. However, the precise parameters that govern the relationship between 471 PIM & BANKS infection of squamous mucosa in various anatomical sites by various HPV types and the development of cancer or non-malignant diseases are still not fully understood. Although data on the host response to normal HPV infection are sketchy, it is likely that similar host immune parameters are relevant to both highand low-risk HPV types (5, for review, and references therein) and there is a body of evidence showing that defects in the innate immune response are what permits a respiratory tract infection by a low-risk HPV type to progress to RRP (6–8); it is likely that similar defects may lead to the progression of genital warts to condylomas and possibly also to persistent infection by high-risk HPV types and risk of cancer. In the past, head and neck cancers have been frequently placed in a single group, whereas the precise tissues that comprise this region are diverse and this is reflected in the frequency of occurrence of different HPV types in different cancers. For instance, HPV-18 is rarely found in SCC of the oro-pharyngeal region, where HPV16 is most common, but more frequently found in SCC of the oral or laryngeal regions. In addition, other high-risk HPV types are, overall, only very rarely found in Head and Neck Squamous Cell Carcinoma (HNSCC) (9), and infections by low-risk HPV types such as 6 and 11 are still rarer. These aspects of HPV infection will be covered in more detail in other sections of this review issue. One of the most significant observations linking infection by HPVs with the development of cervical carcinoma has been the demonstration of the retention of viral DNA in tumour cells, many years after the initial immortalizing events (10, 11). In the overwhelming majority of cases, the cells derived from cervical tumours harbour variable numbers of integrated copies of the viral genome with some portions deleted. Invariably, however, there is retention of the E6 and E7 open reading frames and continued expression of the E6 and E7 proteins (12). That maintenance of the malignant phenotype of these cells absolutely requires continued expression of these two oncoproteins has been demonstrated by experiments where their expression was inhibited in HPV-positive tumour cells causing the cells to undergo apoptosis (13–15), demonstrating unequivocally that regardless of other changes that have taken place at the 472 genetic level in these cells during malignant progression, the interaction between the viral E6 and E7 oncoproteins and cellular targets is absolutely required to maintain this malignant phenotype. Although HPV-16 E6 and E7 have been shown to immortalize primary human tonsillar epithelial cells and so mirror the immortalization results for primary human keratinocytes from genital regions (16), data formally demonstrating that oral SCCs have this same absolute requirement for continued E6 and E7 expression are lacking, due in part to a general lack of suitable HPV-positive tumour cell lines from the head and neck regions. However, it is extremely likely that the same parameters regulating HPVdriven malignancy hold true for both anatomical areas. Low- and high-risk mucosotrophic HPVs infect and replicate in the same general tissues, presumably encountering the same cellular environments and therefore need to overcome the same cellular defences to viral infection. Therefore, it is surprising that considerable differences are observed in their respective pathologies and in their respective cellular targets. In this review, we shall highlight the most well-defined interactions of the viral oncoproteins with their respective cellular targets, which seem to best explain the differences in pathology between the high- and low-risk HPV types. HIGH- AND LOW-RISK HPV TYPES: THE TRANSFORMATION STORY One of the earliest methods used to differentiate between high- and low-risk HPV types was the establishment of transformation assays. Broadly, these assays fell into three categories: transformation of established rodent cells, transformation of primary rodent cells and immortalization of primary human cells. While the cooperative action of the E6 and E7 proteins to immortalize primary human keratinocytes is regarded as the functional ‘gold standard’ of these assays, each system has its own merits and has contributed to our current understanding of E6 and E7 function. As an example, it was the transformation of established NIH3T3 cells with HPV-16 DNA that first demonstrated that the DNA of a highrisk HPV type, found in cervical tumours, had transforming capacity (17, 18). Extension of 2010 The Authors Journal Compilation 2010 APMIS HIGH- AND LOW-RISK HPV TARGETS these assays to primary rodent cells showed that the HPV-16 early region, encoding E6 and E7, together with activated ras, could transform primary baby rat kidney (BRK) cells (19); the principal oncogene responsible in these assays was subsequently shown to be E7 (20, 21) and importantly, that low-risk HPV-6E7 and 11E7 had no detectable transforming activity (21). When the transforming activity of the E6 proteins was assessed using these assays, it appeared to be markedly less efficient than E7 in established cell assays (22, 23) and to have very little activity in BRK cells. But in primary mouse cells, E6 was shown to be almost as efficient as E7 when cooperating with activated ras (24, 25). This species difference probably reflects the efficiency with which the E6 proteins from high-risk HPV types can interact with their relevant cellular partners. The first equivalent assays using human cells showed that primary human keratinocytes, from various genital sites, could be immortalized when transfected with the viral DNA from cancer-associated HPV types without the necessity of co-expressing an activated oncogene such as ras (26–29). These analyses were extended to show that immortalization could be achieved using constructs expressing only the E6 and E7 regions of high-risk HPV types (30, 31), and subsequently, the HPV-16 E6 and E7 proteins have been shown to immortalize human tonsillar epithelial cells (16). It is important to stress that the cells immortalized in these assays are not tumorigenic and to achieve this phenotype, they either require passaging for extended periods, where they are assumed to acquire oncogene-activating mutations, or co-transfection with activated oncogenes (32–34) thus recapitulating aspects of the multistep processes that are believed to govern the malignant progression of cancer in vivo. Essential additions to these experiments have been those that showed that the E6 and E7 from low-risk HPV types had no immortalization capacity (35, 36). The molecular data on the mechanisms of immortalization that have been generated by this approach will be discussed below. In broad terms, what these assays achieved was first to confirm the oncogenic potential of the viral DNA of HPV types that were actually found in cervical tumours. Second, it identified the viral early region, comprising the E6 and E7 2010 The Authors Journal Compilation 2010 APMIS open reading frames as the region responsible. Third, they showed that, at least in a given cellular context, both E6 and E7 could individually transform primary cells and thus paved the way for the first experiments directed at determining the molecular interactions that might explain the oncogenic potential of the high-risk HPVs. These assays, critically, also showed that the E6 and E7 proteins of the low-risk types had no appreciable transforming or immortalizing activity. There are two extensions of these cellular assay systems that have greatly extended our knowledge on how HPVs function in cells. The first is the use of transgenic mouse models to target the expression of viral proteins to specific organs to assess their effects and the second is the development of organotypic raft systems using differentiating human keratinocytes, to examine these same aspects of HPV biology and ultimately to recapitulate the viral life cycle in vivo. These systems will be briefly described below. ASSESSING E6 AND E7 FUNCTIONS IN TRANSGENIC MICE The first meaningful assays demonstrating the same cooperative actions of the high-risk HPV E6 and E7 proteins that were observed in primary rodent cell transformation assays led to the growth of tumours in the murine ocular lens of transgenic mice where the HPV-16 early region, comprising the E6 and E7 open reading frames, was placed under the control of the aA crystallin promoter (37) and in neuroepithelium (38). However, when E6 and E7 were expressed from a keratin 14 promoter, which is activated in cells in the basal layer, tumours arose in the skin and most importantly in the cervix (39–41). These assays showed a striking cooperation between prolonged oestrogen exposure and the presence of the HPV-16 E6 and E7 oncoproteins in the induction of cervical tumours in these mice (41). Indeed a recent study has shown that oestrogen receptor antagonists can inhibit the development of cervical cancer in these transgenic models (42). When the individual contributions of E6 and E7 to skin cancers were assessed in these transgenic systems, E7 functioned more strongly in the stage of tumour 473 PIM & BANKS initiation, whereas E6 was shown to be stronger during the progressive stage of skin tumorigenesis (43). The situation is different in cervical tissues and when the individual contributions of E6 and E7 are assessed in this system, E7 appears to be the dominant oncogene, since when it is expressed alone, after treatment of the mice with oestrogen, it produced reproductive tract carcinomas, whereas the expression of E6 alone did not, unless the mice were treated with oestrogen for an extended period. However, the dual expression of E6 and E7 under the same conditions resulted in larger tumours, indicating that E6 promotes progression (44). In transgenic models of head and neck cancer, as with cervical cancer, E7 appears to be the dominant oncogene (45). Many of the above studies have allowed assessment of the relative contributions made to cancer by some of the cellular targets of E6 and E7 (Tables 1 and 2). Such studies, undertaken with E6 and E7 mutants, have assumed that the same interactions that occur in the mouse system are also valid in human cells. Although the details of such molecular interactions will be discussed in depth below, it is worth noting that the interaction of E7 with pocket proteins has been shown to be necessary, but not sufficient, for its tumorigenicity (45, 46) as is the interaction with the cdk inhibitor p21 (47). Similarly, the interaction with and degradation of p53, though required, is not sufficient to produce either E6-dependent tumours in mouse skin (48) or to explain its oncogenic potential in cervical tissues (49) and the presence of a PDZ-binding motif has been shown to be absolutely required for the ability of high-risk E6 to drive epithelial hyperplasia (48, 49), although this ability is, by itself, insufficient for E6 tumorigenicity. ORGANOTYPIC RAFT SYSTEMS In contrast to other DNA tumour virus types, such as the adenoviruses and polyoma viruses, the early days of papillomavirus research were hampered by a lack of cellular systems capable of infection by HPVs, which inevitably led to the use of the transformation and molecular strategies that have been discussed in this review. Productive though these strategies were in terms of defining the oncogenic properties of HPVs, they gave little information regarding the normal papillomavirus life cycle. The first Table 1. Cellular targets for the E7 proteins from high- and low-risk HPV types. Listed are those cellular targets that the authors believe are most relevant to separate the oncogenic as opposed to non-oncogenic properties of the E7 proteins from the two HPV types HPV E7 interactions: functional consequences Cellular target pRB p107 p130 p21 p27 Cyclin A Cyclin E TBP P300 ⁄ CBP ) o High-risk Low-risk Proteasome-mediated degradation De-repression of cell cycle genes Cell survival Modulation of differentiation Proteasome-mediated degradation of p107 pRB and p130: NO Overrides cell cycle inhibition p21: weakly overrides cell cycle inhibition? p27? No Overrides cell cycle inhibition Transcriptional modulation ? Modulation of acetylation and histone Modulation of acetylation and histone modification modification-WEAK MPP2 Transcriptional modulation No IGFBP-3 Modulation of insulin signalling ? Mi2 Recruitment of HDAC1 and 2 modulation of No histone acetylation and transcription NuMA Dissociation of NuMA ⁄ Dynein complex Dissociation of NuMA ⁄ Dynein complex Mitotic defects Mitotic defects p600 Transformation, anchorage-independent growth ? HPV, human papillomavirus; NuMA, nuclear mitotic apparatus protein 1. 474 2010 The Authors Journal Compilation 2010 APMIS HIGH- AND LOW-RISK HPV TARGETS Table 2. Cellular targets for the E6 proteins from high- and low-risk HPV types. Listed are those cellular targets that the authors believe are most relevant to separate the oncogenic as opposed to non-oncogenic properties of the E6 proteins from the two HPV types HPV E6 interactions: functional consequences Cellular target p53 High-risk Inhibition of transcription Proteasome-mediated degradation Overriding cdk inhibitors and apoptosis Bak Proteasome-mediated degradation Inhibition of apoptosis myc Transcriptional activation of hTERT Co-immortalization of primary cells E6AP Mediates E6 stability Proteasome-mediated degradation UbE3 ligase for substrate targeting E6BP ⁄ ERC55 Binding yes; modulation of calcium-mediated differentiation? P300 ⁄ CBP Binding to three domains of p300 ⁄ CBP Inhibits intrinsic transactivation function; modulation of acetylation PDZ proteins Proteasome-mediated degradation, loss of cell polarity, changes in actin kinetics, modulation of signal transaction hTERT Activation of telomerase Tyk2 Interference with host immune system by blocking interferon-a activation of Jak ⁄ STAT signalling hAda3 Proteasome-mediated degradation Abrogation of p53 ⁄ p14Arf pathway HPV, human papillomavirus. organotypic raft experiments undertaken examined the stratification of primary keratinocytes immortalized by high-risk HPV DNA allowing the analysis of the phenotypic changes that occur during differentiation. These experiments showed that the changes in cellular differentiation that occurred with extended passage mirrored the cellular phenotypes typical of the progression of pre-malignant to malignant lesions observed in patients and thus linked the effects observed during real cancer progression with the effects seen when immortalized keratinocytes are grown in normal culture (50). These experiments, although useful, failed to recapitulate the viral life cycle as no viral particles were synthesized. In the first study demonstrating virion production, cells were treated with phorbol ester to induce differentiation of an HPV-31b-containing keratinocyte line grown in raft culture (51), a strategy that allowed some of the first analyses of the link between cellular differentiation and changes in viral transcription to be made (52). These initial studies with HPV31b were ultimately repeated with HPV-18 to 2010 The Authors Journal Compilation 2010 APMIS Low-risk Inhibition of transcription Proteasome-mediated degradation Overriding cdk inhibitors and apoptosis Proteasome-mediated degradation Inhibition of apoptosis-WEAK ? Proteasome-mediated degradation? UbE3 ligase for substrate targeting Confirmed for HPV-11 E6 only ? Binding to one domain of p300 ⁄ CBP Consequences? No No Weak interaction, modulation of host immune response? No produce infectious virions (53) and subsequently with HPV-16 (54). Interestingly, collagen raft systems were also shown to function for HPV11-positive laryngeal cells (55). However, the first study that allowed a comparison between the biology of high- and low-risk HPV types in raft cultures also showed that, although HPV11 cannot immortalize keratinocytes, their lifespan was considerably extended, and in addition the differentiation pattern of the raft cultures was also altered, albeit to a lesser degree than seen with HPV-31 (56). While no major changes were seen between HPV-11-containing and normal keratinocytes in the levels of cell cycle-relevant proteins such as cyclins or cdk inhibitors, a major difference was the lack of repression of interferon-regulated genes that is seen with high-risk HPV types, although these comparisons were made for cells in monolayer rather than under conditions of differentiation. While global changes in cellular gene expression have been assayed by microarray analysis for highrisk HPV type E6 and E7 proteins (57), this type of comprehensive analysis has not been 475 PIM & BANKS undertaken for low-risk type E6 and E7. Subsequent studies have obtained viable organotypic raft systems capable of recapitulating the HPV11 life cycle, but only in cells that have been immortalized by ectopic expression of TERT, the catalytic subunit of telomerase (58). It is evident that there are a number of gaps in the use of such systems regarding the analysis of lowrisk HPV life cycles, but it is anticipated that recent studies describing efficient production and passaging of HPV-18 in raft culture systems (59, 60) should pave the way for a more comprehensive comparative analysis between the life cycles of high- and low-risk HPV types. INTERACTIONS WITH CELLULAR TUMOUR SUPPRESSORS Some of the first molecular explanations for the malignant potential of the high-risk HPV types, 16 and 18, came with the observations that their E6 and E7 oncoproteins could interact with the key cellular tumour suppressors p53 and pRB. p53 is a multifunctional transcriptional modulator and inducer of apoptosis. In response to DNA damage, nucleotide depletion or hypoxia, it becomes activated by acetylation and phosphorylation, functioning as a nuclear transcription factor to induce genes involved in cell cycle inhibition or apoptosis, or it can induce apoptosis more directly by interacting with proteins in the cytoplasm at mitochondrial sites. Its multiple functions have been studied extensively for more than a quarter of a century (61, for review) and one attractive model to explain the oncogenicity of high-risk HPV types might be its functional abrogation by papillomavirus E6 proteins, thereby allowing mutations that activate cellular oncogenes to pass unchecked. Similarly, pRB is also a key regulator, whose interaction with the members of the E2F family of transcription factors regulates both cell cycle progression and apoptosis. As with p53, pRB has been extensively studied over a period of many years (62, 63 for reviews) and it is clear that one model to explain the transforming capacity of the E7 proteins of high-risk mucosal HPVs in established and primary cells might be their abrogation of pRB function. Both p53 and pRB mutations are common in many types of 476 cancer, but occur very rarely in early-stage cervical cancers, inviting the speculation that their functional abrogation by the E6 and E7 oncoproteins from high-risk mucosal HPVs is, to a degree, functionally equivalent to mutation in other cancer types. Because of the functional complexity and importance of these two key cellular proteins, their interaction with the viral oncogenes will be described below. E7 AND ‘POCKET PROTEINS’ Analysis of the amino acid sequence of the E7 proteins from high-risk mucosal HPVs shows regions of conservation with both adenovirus E1a and SV40 large T antigen. It was this observation that led to experiments showing that HPV-16 E7 could interact with pRB (64) and that the E7 proteins from low-risk types could also interact with pRB, but less efficiently (65, 66). Subsequently, it was shown that high-risk, but not low-risk, E7 could induce the proteasome-mediated degradation of pRB (67). Although the precise pathway involved in E7directed degradation of pRB remains to be mapped, recent studies have begun to unravel its complexity (68, and references therein). It has also been shown that pRB is ubiquitinated by an HPV-16 E7-cullin 2 ubiquitin ligase complex (69), and so E7 may function as a ligase adapter to target pRB by a mechanism that is analogous to the way in which high-risk E6 functions towards p53, as described below. The other pocket protein family members p107 and p130 were also shown to be targeted by HPV-16 E7 (70–72). Interestingly, the degradation of p130, but not of either pRB or p107, has also been recently shown for the E7 proteins from low-risk HPV types (73) demonstrating at least some conservation of function between the E7 proteins from high- and low-risk HPV types. E7 interacts principally with the active, unphosphorylated form of pRB (74) and in broad terms, this leads to the de-repression of E2Frepressed genes, whose expression is required for cell cycle progression from the G1 to the S phase. By analogy with the conserved regions 1 and 2 (CR1: amino acids 37-49; and CR2: amino acids 117-137) of adenovirus E1A, the amino terminus of E7 can be broadly divided into conserved domains 1 and 2 (CD1: amino 2010 The Authors Journal Compilation 2010 APMIS HIGH- AND LOW-RISK HPV TARGETS acids 1-15; CD2: amino acids 17-37) and it is the LXCXE motif in CD2 of E7 that interacts with the pocket domain of pRB (75, 76). The key feature of the E7–pocket protein interaction is that it leads to deregulation of the normal cell cycle and loss of checkpoint controls (77, 78); cells that have divided upwards from the basal layer would normally, permanently exit the cell cycle and start to differentiate. HPVs must re-initiate or maintain a functional cell cycle in these cells to have a supply of proteins required for S-phase progression, and to amplify viral DNA. HPV E7 therefore is able to uncouple keratinocyte differentiation from cell cycle progression and it is a central tenet of the current model for HPV-induced transformation that this unscheduled DNA synthesis is what activates the cellular apoptotic pathways by a mechanism that has been termed the ‘trophic sentinel response’ (79) and this response is inactivated by the high-risk HPV E6 proteins. Low-risk E7s do not appear to activate this response despite their interaction with pocket proteins. pRB-INDEPENDENT FUNCTIONS OF THE E7 PROTEINS The E7–pRB interaction and its consequences have been thoroughly reviewed (80, 81, for reviews), but there are other interactions between E7 and cellular proteins that contribute to its driving of, or inhibition of withdrawal from, the cell cycle, as mutations in other regions of E7, particularly the carboxy-terminal zinc-binding finger, can also abolish transforming ability (82, 83). For example, E7 binds to Mi2, a component of histone deacetylase (HDAC) NURD complexes (84), and this interaction is thought to mediate the interaction between high-risk E7 and HDACs 1 and 2 – an interaction that may allow E7 to modulate histone modification and transcription of cellular genes relevant to cell cycle deregulation (85) or to immune evasion (86). Another example is that during keratinocyte differentiation, loss of contact with the basal membrane is accompanied by increased levels of the cyclin ⁄ cdk inhibitors p21 and p27 (87, 88), and increased levels of both p21 and p27 are found associated with specific cyclin ⁄ cdk complexes (88, 89). High-risk E7 can overcome the cell cycle inhibition 2010 The Authors Journal Compilation 2010 APMIS mediated by both of these proteins. In the first instance, high-risk E7 can interact with p21 and block its ability to inhibit cyclin E ⁄ cdk2 activity (90, 91); low-risk E7 also appears to interact with p21, but appears to have a reduced ability to relieve p21-mediated cell cycle arrest (77). A similar mechanism appears to operate for E7 relief of p27 inhibition (92). High-risk E7s also appear to help drive cell survival pathways by increasing Akt phosphorylation, both by pRBdependent (93) and -independent pathways (94). The binding of high-risk E7 protein through its amino terminus with pRB-associated factor p600 has been shown to be independent of any interaction between E7 and pocket proteins, and appears to contribute to the transforming capacity of E7 and the ability of tumour cells to grow anchorage-independently (95). In addition to these functions, E7 proteins have properties that modulate cellular transcription. For example, both low- and high-risk E7 proteins have been shown to interact with the cellular histone-modifying machinery, such as pCAF, in this case disrupting its acetyl transferase functions, resulting in reduced transformation and transactivation (96), and both low- and high-risk E7 interact with p300, also disrupting its transactivating capacity (97). E7 has also been shown to associate with MPP2, a member of the forkhead transcription factor family (98), and AP-1 (99) as well as the basal transcription machinery (100, 101). The finding that high-risk E7 can interact with insulin-like growth factor-binding protein 3 (IGFBP-3) (102) and accelerate its proteolytic turnover (103) links high-risk HPV to modulation of the insulin signalling pathway, and it has also been shown that IGFBP-2 and 3 levels are increased in HPV-16 E7 expressing cells under low growth factor conditions in an NFjB-dependent manner (104), the situation seen when basal cells divide upwards from the basal layer. This observation may also be linked to the ability of infected cells to evade the host immune system. It is the link between high-risk HPV E7 and the induction of aneuploidy that has become the focus of attention more recently (105, for review). It has been known for several years that expression of the E7 oncoprotein from high-risk HPV types caused chromosomal changes (106), a property that seems not to be 477 PIM & BANKS shared with the low-risk HPV types (107, 108). Amongst the chromosomal damage observed in cells expressing HPV oncogenes, perhaps the most prominent are multipolar mitoses, due to aberrant numbers of centrosomes resulting from centrosome duplication errors (109, 110). The molecular mechanism that leads to such defects has been shown to be in part due to a pRB degradation-dependent increase in CDK2 activity (111) and partly due to an association with gamma-tubulin (112). Although high-risk E7 has been shown to cause delocalization of dynein from mitotic spindles (113), this seems not to be directly associated with the induction of mitotic defects. However, there is an interaction with nuclear mitotic apparatus protein 1 (NuMA), an interaction that is shared with the E7 protein from low-risk HPV types and which results in a prometaphase delay (114). Aneuploidy that results from such mitotic defects is considered to play a significant role in DNA damage of the type that might push HPVimmortalized cells towards malignancy and would be complemented by those aspects of E6 function that have also been shown to lead to mitotic abnormalities (108, 115). There are, however, potential problems with such a model. First, the effects demonstrated occur rapidly, whereas in our current understanding of HPVdriven malignancy, the effects that occur during persistent infection are on a scale of years. Second, the viral E2 protein has been shown to inhibit the E7-dependent centrosome duplication errors by direct interaction with E7, rather than by any transcriptional-repressive means (116) and third, the generation of at least some mitotic defects is shared by the E7 proteins from low-risk HPV types because of their interaction with NuMA (114); if low-risk types induce such defects why are they not associated with malignancy? However, this remains an attractive model for HPV-induced DNA damage if one considers that levels of E6 and E7 are likely to be very low during normal, even persistent infection, but that at some stage the viral E2 protein loses the ability to repress the viral early promoter and E6 and E7 levels rise to a high enough level to perturb centrosome duplication. Aneuploidy that results from these errors might activate DNA damage repair mechanisms that increase the risk of viral integration, a model that has been proposed in the 478 past (117), and integration of the viral genome is an event that occurs frequently during cervical cancer progression. Indeed, recent evidence has shown that HPV-31 E7 interacts with ATM kinase and leads to the activation of downstream pathways such as CHK2, BRCA1 and NBS1 (118), and HPV-16 E6 has been shown to dysregulate CHK1 activity (119). Although activation of these pathways is thought to be required for viral genome amplification upon host cell differentiation, it is possible that their perturbation in the context of aneuploidy-driven DNA damage might have more serious consequences. E6 AND p53 HPV-16 and 18 E6 were shown to interact with the cellular tumour suppressor p53 (120) and then subsequently to direct its degradation (121). While the intracellular levels of p53 are normally regulated by the cellular ubiquitin ligase hdm2 (122, 123), high-risk HPV E6 proteins interact with p53 and direct its degradation by recruiting a different cellular ligase, E6AP (124, 125). Subsequent studies showed that the E6 proteins from low-risk HPV types could also interact with p53, but failed to increase its rate of turnover (126, 127), showing that low-risk E6 employs a different mechanism for the abrogation of p53 function, most likely by reducing its reported ability to both repress TATA-containing promoters (128) and to activate p53-responsive promoters (25, 129). These early studies suggested that the E6 from low-risk HPV types failed to direct the degradation of p53 because they were unable to interact with and recruit E6AP, but more recent data have changed this model for low-risk E6 function as will be discussed later on in this review. Studies had already suggested that the region of E6 involved in binding p53 might be separate from that involved in inducing its degradation (126, 129), but conclusive data showing precisely which regions of high-risk E6 bind to p53 and which bind to E6AP have been lacking, with many publications showing conflicting results. However, when looking at the regions of p53 that interact with E6, an explanation for the functional difference between the E6 proteins from high- and low-risk types came from studies 2010 The Authors Journal Compilation 2010 APMIS HIGH- AND LOW-RISK HPV TARGETS showing that the E6 from high-risk types could bind to p53 at two regions, a carboxy-terminal region (amino acids 376-384) and a core region (amino acids 66-326), whereas the E6 from lowrisk types bound to p53 only at the carboxy terminal region (130). More recent data suggest that the ability of the E6 proteins from low-risk HPVs to interact with p53 and modulate some of its functions occurs by sequestering it in the cytoplasm (131). Given that some of the functions of p53 also occur in the cytoplasm, and that the E6 proteins from high-risk types export p53 from the nucleus to the cytoplasm when directing its degradation (132), it would be interesting to re-assess the effect of E6 from low-risk HPV types on these functions. Another aspect of HPV E6 function independent of its direct interaction with p53, but highly relevant to the p53 pathway, is p53 acetylation. High-risk, but not low-risk HPV E6 interacts with hAda3, a component of histone acetyltransferase complexes that is involved in driving the p53 ⁄ p14Arf pathway by acetylation of p53. High-risk E6 directs its degradation in an E6AP-dependent manner thereby inhibiting p53-mediated senescence (133, 134). However, both high- and low-risk HPV E6 proteins seem capable of binding to CBP ⁄ p300 and this interaction has been shown to prevent the acetylation of p53, independently of any ability to direct E6AP-mediated degradation of p53, thereby inhibiting its ability to transcriptionally activate promoters (135). This observation suggests a higher degree of functional conservation between the E6 proteins of high- and low-risk HPV types, with respect to p53 function, than was previously considered. increase its rate of proteolytic turnover through the E6AP ⁄ proteasome pathway (138, 139) and it is significant that for cutaneous HPV types, this interaction with Bak is the only apoptosisrelevant pathway so far clearly defined (140). High-risk E6 binds strongly, but, low-risk E6 only weakly to p300 ⁄ CBP (141, 142). Only highrisk E6 proteins bind to E6BP ⁄ ERC-55 (143), MCM7 (144) and c-Myc (145). Both bovine papillomavirus type 1 (BPV-1) and high-risk, but not low-risk, HPV E6 proteins bind to Paxillin (146), a component of the focal adhesions that attach keratinocyte basal cells to the basal membrane and which is required for driving integrin-mediated proliferation and survival signalling through focal adhesion kinase (FAK). However, while BPV-1 E6 degradation of Paxillin is required for its transforming capacity (147) the significance of the interaction with high-risk HPV E6 is likely different because in both HPV-positive carcinoma cells and in HPV-immortalized keratinocytes, paxillin levels and FAK-driven tyrosine phosphorylation are increased rather than decreased (148). Of relevance to the way in which HPVs can evade host immune systems was the observation that high-risk E6 could inhibit the interferon-a activation of the Jak-STAT pathway by interacting with tyrosine kinase Tyk2 – an interaction that was observed to occur less strongly for low-risk E6 types (149). Finally, there are those cellular interactions that are relevant to the reactivation of telomerase in infected cells and a growing list of cellular proteins containing PDZ domains, understanding the consequences of which may be more relevant in modelling immortalization ⁄ transformation ⁄ cancer progression functions. Both of these will be discussed in detail below. P53-INDEPENDENT FUNCTIONS OF E6 E6 AND TELOMERASE ACTIVATION There are several lines of evidence, based on analysis of the function of E6 mutants in cellbased assays, that point to the involvement of E6 in other aspects of cell biology, independently of its interaction with p53, that contribute towards its oncogenic potential (25, 136, 137). Indeed, a large number of cellular binding partners have been described for E6 that might contribute to its oncogenic character, some of which are shared by the E6 proteins from low-risk HPV types. High- and low-risk E6s bind to Bak and 2010 The Authors Journal Compilation 2010 APMIS Isolated primary cells undergo a finite series of population doublings before embracing death (150) by the activation of senescence pathways. Those cells that survive this ‘crisis’ stage and continue to grow are found to have activated telomerase, the enzyme complex that maintains the telomeric repeats at the ends of chromosomes (151). Typically, the mechanism involves transcriptional activation of the gene for hTERT, the catalytic subunit of the telomerase 479 PIM & BANKS complex. Tumour cells also achieve immortality in a great number of cases by a similar mechanism (152). When primary human keratinocyte immortalization assays with high-risk HPV E6 and E7 were first set up, it was realized that their telomere lengths were restored in contrast to non-immortalized cells (153) and that the reason for this was due to reactivation of telomerase activity (154). The mechanism by which telomerase becomes activated during immortalization by E6 and E7 is complex, but is becoming clear. For instance, the use of E6 mutants shows that the mechanism is independent of the ability of E6 to bind and direct the degradation of either p53 (154, 155) or PDZ proteins (49), but involves transcriptional upregulation of the hTERT promoter by a mechanism involving SP1 binding and down-regulation of p300 (156) and depends on Myc (157). Myc is found bound directly or indirectly to high-risk E6 in complexes at the E-boxes within the hTERT promoter (158), an observation that appears to be at odds with the original finding that suggested that Myc was targeted for proteasome-mediated degradation by high-risk E6 in an E6AP-dependent manner (145). In addition, a recent report has demonstrated that E6 can itself also make a direct interaction with the telomerase complex (159). The question of whether binding to the ubiquitin ligase E6AP is required seems to be controversial, some reports showing it to be required (160) and that E6AP-dependent degradation of the transcriptional repressor NFX1-91 is necessary for hTERT activation (161), whereas other reports seem to show no requirement for high-risk E6 to interact with E6AP to activate hTERT (162). While E7 is by itself unable to activate telomerase, it is clear that it augments the activity of E6 by transcriptional activation of the hTERT promoter in a pRBdependent manner (163). Evidently, activation of telomerase appears to be an important facet of high-risk HPV-induced immortalization and an aspect of the viral properties not shared with low-risk types; however, how this relates to the normal viral life cycle and why low-risk HPV types seem not to activate hTERT remain to be clarified. It may be a function related to episomal maintenance because telomeric proteins have been shown to be involved in the episomal maintenance of Epstein–Barr virus origins of replication (164). 480 OTHER HIGH-RISK VS LOW-RISK INTERACTIONS From the data presented above, it should be clear that many of the interactions with cellular targets that are made by the E6 and E7 proteins from the high-risk mucosotrophic HPV types are shared by their counterparts from the lowrisk group, albeit to a generally lesser degree. A plausible argument for the difference in their oncogenic potential could be that it is due to these differences in the efficiency of their targeting of the same set of cellular proteins. Data that might support this come from the observation that in extremely rare cases, RRP can have lung involvement, and that a surprisingly high proportion of these lung-involvement cases can result in lung cancer. Analysis of the HPV in these cases shows that the virus has undergone substantial mutation, such as amplification of the viral URR, which might lead to much higher levels of E6 and ⁄ or E7 being expressed and thus showing that given the right circumstances, in the right tissue type, even low-risk HPV types have oncogenic potential (B. Steinberg, personal communication). However, there is a set of interactions that is exclusive to only the E6 proteins of the high-risk HPV types and these will be discussed below. HIGH-RISK E6 PROTEINS AND PDZ DOMAINS The extreme carboxy termini of the E6 proteins from all of the high-risk human mucosotrophic papillomaviruses contain a consensus sequence that is able to bind to PDZ domains (PSD95 ⁄ Dlg ⁄ Zo-1). The PDZ-binding sequence is absent from the E6 proteins of the low-risk HPV group and both this sequence and the ability to bind PDZ domains are therefore molecular hallmarks of the E6 proteins from oncogenic HPV types. These domains, 80-100 amino acids in length, serve as docking modules for a large variety of different cellular proteins, including signalling molecules (165, for review). A subset of these proteins, the Membrane Associated Guanylate Kinases (MAGUKs), has multiple PDZ domains and can serve as molecular scaffolds to conjugate incoming signals at the plasma membrane and transmit them to 2010 The Authors Journal Compilation 2010 APMIS HIGH- AND LOW-RISK HPV TARGETS multiple downstream effector pathways. These PDZ proteins are found at a variety of cell junctions and synapses, where they are also involved in defining and maintaining cell polarity, as well as themselves being involved in nuclear-cytoplasmic shuttling and signalling, and mediate the kinetics of actin polymerization and depolymerization at the leading edge during cell migration. Most importantly, loss or mislocalization of these complexes during malignant progression of cancer correlates with the loss of cell polarity and invasiveness that is generally observed with metastasis. Because of the large number of identified PDZ proteins and the roles that they play in almost every conceivable aspect of cell biology, it can be seen that the E6 proteins from oncogenic HPV types have the potential of modulating a large variety of cellular pathways. However, so far, although the list of PDZ domain partners for E6 is growing, it is also evident that there is a set of defining parameters that govern which PDZ domains may be bound by E6 and which not. In particular, the efficiency with which a particular E6 may direct degradation of a given PDZ protein may be defined by its exact PDZ-binding motif (166, 167), and the observation that even for proteins with multiple PDZ domains E6 seems to interact with only one PDZ domain per target protein (167–171) suggests selectivity in targeting. The first identified PDZ domain-containing target to bind to E6 was Dlg, the human homologue of Drosophila discs large (172), and E6 was subsequently demonstrated to direct its degradation along a proteasome-mediated pathway (173). Other studies identified hScrib as a target for E6-directed degradation (174) and subsequently MAGI-1, -2 and -3 (169, 175), MUPP1 (168) and many others (1, 176, for review). Many PDZ proteins as well as the MAGUK targets mentioned above localize to sub-membrane sites where they play a role in maintaining junction integrity and cell polarity. It has been regarded as central to the philosophy of the current model for cervical cancer progression, that degradation of these proteins relates to metastasis. However, at least in the case of Dlg, if cells derived from cervical tumours are treated with proteasome inhibitors, the Dlg that is rescued is predominantly localized in the nucleus (177), suggesting that it is a nuclear pool of Dlg 2010 The Authors Journal Compilation 2010 APMIS that is targeted by high-risk E6. As studies have also shown that nuclear forms of Dlg are phosphorylated by cyclin-dependent kinases and preferentially targeted by E6 (178, 179), this suggests that nuclear forms of Dlg may be playing an antiproliferative role and that in HPV-positive cervical tumour cells, this is being inhibited, in part, by its E6-directed degradation. This observation does not by any means rule out the possibility that membrane-localized forms of Dlg, or other PDZ domain-containing proteins, may be targeted during the normal viral life cycle; a speculative model might be that by loss of these proteins and a corresponding loss of polarity, infected basal cells might divide in a horizontal rather than a vertical orientation, thereby expanding the population of infected cells that are still attached to the basal membrane. This kind of model suggests that highrisk E6s may target a specific set of PDZ proteins in a particular subcellular localization, that are involved in maintaining cell polarity during the normal viral life cycle, but target PDZ proteins in a different subcellular localization during malignant progression. That MAGUK proteins mediating cell junction integrity might be the target of choice for the normal viral life cycle is supported by the finding that these proteins are generally targeted by high-risk mucosal viruses, whether or not there is any evidence for their nuclear localization. Dlg and hScrib are members of a complex found at adherens junctions (180, for review). PATJ, another target for high-risk E6 is found localized to tight junctions (181) as is MUPP1 (182), and the MAGI-1, 2 and 3 proteins. Although the MAGI proteins are implicated in a wide variety of cellular activities such as the MAGI-1 regulation of b-catenin signalling (183) and the MAGI-2 regulation of the PTEN pathway (184), they are also membrane localized for many of their functions. A recent study of another high-risk mucosal papillomavirus type, rhesus papillomavirus type 1 (RhPV-1), shows the presence of a PDZ-binding motif on the carboxy terminus of the E7, rather than the E6 protein. In this case, the preferred target for degradation is Par-3, a component of the apical polarity complex (185). All these data suggest that high-risk HPV types generally target polarity-regulating complexes as part of their normal life cycle. While it is currently unclear what roles these interactions play, there is good 481 PIM & BANKS evidence for its essential requirement because removal of the PDZ-binding motif, in the context of the whole viral genome, results in the loss of episomal maintenance (186). New PDZ targets of the E6 oncoproteins from high-risk HPV types are continually being identified and of particular note are a subset of tyrosine phosphatases. Given their involvement in the inhibition of signal transduction through receptor and non-receptor tyrosine kinases from membrane-proximal sites, driven by external stimuli, a reduction in their activity by E6-directed degradation might be expected to drive signal transduction pathways that relate to cellular proliferation. The first phosphatase to be identified as a target was PTPH1, also known as PTPN3 (187, 188), where its degradation by oncogenic E6 was shown to correlate with reduced growth factor requirements. Perhaps more important, due to its reported relevance in head and neck cancers, is the protein tyrosine phosphatase PTPN13 (189). In this case, degradation of the phosphatase (also known as PTPL1, PTP-Bas and FAP-1) appears to be relevant for anchorage-independent growth, where it results in increased signalling along the mitogen-activated protein kinase pathway (190). Although these studies used oral cells for analysing E6 function, it is most likely that E6-mediated disruption of tyrosine phosphate function plays a role in the life cycle of these high-risk HPVs in cervical keratinocytes. Some of the cell junction-localizing substrates of high-risk E6 also have consensus sites for tyrosine kinases and are likely to be tyrosine phosphorylated. We speculate that E6 might therefore be exerting a two-pronged attack on some of its substrates, by degrading a subset of tyrosine phosphatases, and thus enhancing upstream kinase pathways for these MAGUK targets, altering their phosphorylation and ⁄ or cellular localization and, as a result, also possibly altering their susceptibility to E6-mediated degradation. That this aspect of the biological properties of E6 is essential for its oncogenicity is clearly demonstrated by the finding that in the transgenic mouse models discussed above, E6 mutants with deletions in the PDZ-binding motif are unable to induce hyperplasia (49) and E6 lacking a PDZ-binding motif cannot cooperate with E7 to immortalize human tonsillar epithelial cells (189). 482 THE E6 PROTEINS FROM LOW-RISK HPV TYPES; POTENTIAL LIGASE INTERACTIONS Although the presence of a PDZ-binding motif on the carboxy terminus of high-risk HPV E6 proteins and its absence from the low-risk HPV E6s might be regarded as a defining means of separating their relative risk factors, and while the E6s from low-risk HPVs were originally regarded as being unable to recruit ubiquitin ligases for degrading cellular targets, the cloning of the last six amino acids from a high-risk E6 onto the carboxy terminus of a low-risk E6 fully enables it to direct the degradation of certain cellular targets (191). This study demonstrated not only that E6 from low-risk HPVs are able to conjugate to cellular proteolytic systems but also that the way in which individual cellular proteins are targeted may be via different degradatory systems, or via different ubiquitin ligases. The human tumour suppressor p53 has two common polymorphic forms encoding either arginine or proline at amino acid position 72 (192), and previous studies have hinted tantalizingly at a modest ability for HPV-11 E6 to degrade at least the Arg form of p53 (193). Interestingly, a more recent study has demonstrated an interaction between HPV-11 E6 and E6AP (194), suggesting the means by which HPV-11 E6 may do this. The story of ubiquitin ligase recruitment is, however, far from complete. Previous studies have suggested rather strongly that the high-risk HPV E6 proteins may recruit ligases other than E6AP to direct the degradation of certain cellular targets (166, 191) and several additional studies have supported this. First, synthetic peptides that inhibited the binding and degradation of some of E6s targets were unable to block the degradation of others (195) and second, certain cellular targets were found to be still degraded when incubated together with E6 after translation in vitro in wheat germ extract, which lacks E6AP, or when rabbit reticulocyte lysate had been immunodepleted of E6AP (196). More recently, the observations that high-risk E6 can direct p53 degradation without inducing its ubiquitination (197) and that E6-directed degradation of some substrates can occur in E6AP-null mouse fibroblasts (198) suggest that we are only just beginning to understand the complex series of 2010 The Authors Journal Compilation 2010 APMIS HIGH- AND LOW-RISK HPV TARGETS interactions by which the high-risk E6 proteins may target different cellular substrates. The search is on for new interacting ubiquitin ligases and ubiquitin pathway components that may be involved in substrate targeting. New data have recently shown that the high-risk E6 protein is in fact stabilized by E6AP (199) and that a novel ubiquitin ligase, EDD, interacts with E6 and has been shown to regulate the stability of E6AP (V. Tomaić, personal observations) and the ubiquitin-specific peptidase USP15 has also been shown to be involved in the regulation of E6 stability (200). Doubtless new ubiquitin ligase discoveries are on the horizon and will help to unravel the complex set of interactions and pathways defining the molecular parameters that govern the targeting of the cellular substrates of E6. OTHER HIGH-RISK SPECIFIC FUNCTIONS High-risk and low-risk mucosal HPV types express their E6 and E7 open reading frames differently. For HPV-6 and 11, E6 and E7 have two separate transcriptional start sites, whereas for types 16 and 18, there is only one, so that E6 and E7 are transcribed from the same promoter. An examination of the transcriptional patterns from HPV-positive cell lines has shown a complex pattern of polycistronic mRNAs that can be multiply spliced to encode a range of viral early proteins (201). For types 16 and 18, the presence of a consensus splice donor site within the body of the E6 open reading frames of such early transcripts means that, in addition to other open reading frames that might be encoded, removal of an intron within the body of the E6 open reading frames can generate one or more small truncated E6 transcripts known as E6*. Where these introns terminate and the number of potential E6* transcripts that can be encoded in this way is defined by the siting of the downstream splice acceptors, but invariably these run back into a different, intron-derived reading frame, so that E6* transcripts encode a tail of amino acids derived from neither E6 nor E7, and terminate at the first available stop codon. This pattern for the siting of the first splice donor and acceptor is strongly conserved among most, but not all, high-risk mucosal 2010 The Authors Journal Compilation 2010 APMIS HPV types; HPV types 52, 59 and 67 lack these strongly conserved donor ⁄ acceptor patterns (202) and it has not been shown whether or not they can make E6* transcripts. However, these conserved splicing modules within the E6 open reading frame are found neither in low-risk HPV types nor in cutaneous HPVs. It has been an open question whether these truncated E6 forms are ever translated to express the E6* protein, although the majority of early transcripts found in HPV-positive cell lines are spliced and encode E6* (11, 203). Several in vitro studies have suggested that E6* proteins could bind to their full-length E6 counterparts and were able to inhibit the E6-directed degradation of p53 (204, 205). Additionally, data suggest its involvement, together with full-length E6 in modulating components of the extrinsic death pathway (206). The most recent data on E6* function suggest that it can decrease the half-life both of PATJ (207) and Dlg when expressed by itself at high levels, and may also affect the stability of other PDZ proteins (202). Compelling evidence suggests that it provides a helper function for full-length E6 in activating the degradation of differentially phosphorylated targets, because Dlg phosphorylated along the p38 MAPK pathway, but not along the SAPK ⁄ JunK pathway, is markedly more susceptible to degradation by E6 when co-expressed with E6* than it is to degradation induced by either protein alone (202). It is unlikely that the E6* protein reaches high enough levels in vivo to activate degradation by itself; although it is not an intrinsically unstable protein, it is expressed at very low, although detectable, levels in HPVpositive HeLa cells (D. Pim, personal observations). Clearly, existing in vitro models for HPV transformation do not predict a major role for the E6* proteins; however, it is quite possible that in vivo, E6* augments the effect of full-length E6 by modulating diverse upstream signalling pathways. CONCLUSIONS Any contemporary model that seeks to differentiate between the malignant potential of highand low-risk HPVs in terms of their molecular interactions is faced with a number of problems. One such problem is the relative lack of 483 PIM & BANKS available data for the molecular interactions made by the E6 and E7 proteins of the low-risk types. Regardless of the clinical implications of infection by the low-risk types, their lack of association with malignant disease has inevitably made them less attractive fields in terms of funding and research. Similarly, the low-risk types have failed to produce viable working models, or yield significant data in key research systems such as cellular transformation models and in raft culture, leaving substantial gaps in our understanding. Nevertheless, where molecular studies have been possible, the low-risk types have proved to be every bit as interesting to study as the high-risk. So how can we distinguish between these viral types? Are there life cycle differences that correlate with differences in oncogenicity? Clearly, the pathology of these groups is different, with the high-risk mucosal HPVs producing planar warts in genital tissues, as opposed to the papillomas produced by low-risk types, and yet these differences are not invariably reflected in the sequential pattern of expression of their various proteins or the tissue layers where viral genome amplification takes place during the viral life cycle. An example of such analysis shows that low-risk HPV-11 appears strikingly similar to high-risk HPV-16 (208). Where low-risk HPV E6 and E7 interactions with cellular proteins have been studied, the consensus has been that many interactions that are seen with the highrisk oncoproteins either do not occur, or are very much weaker; this itself could partly explain their lack of correlation with malignancy. However, perhaps the two most significant differences between high- and low-risk HPV types are the ability of the high-risk E7 proteins to induce chromosomal damage by interfering with the centrosomal duplication pathway and the interactions between the highrisk E6 proteins and cellular targets containing a PDZ domain. Of relevance to this latter interaction, it is probable that malignant transformation by the high-risk HPVs may arise under conditions of abortive infection and it has been suggested that the transition zone, where most cervical carcinomas originate, may be of a tissue type that is less competent to support viral replication (209). It is of note that the two most prevalent high-risk types, HPV-16 and HPV-18, produce tumours of different cytology, SCCs by 484 HPV-16 and adenocarcinomas by HPV-18 and in distinct regions of the uterine cervix. Given that the transition zone delineates these two regions, it may well be that infection by highrisk HPV types at this transition zone has a higher risk of malignant progression and that the differences in the targeting specificity of polarity-determining PDZ proteins by the E6 oncoproteins of these two HPV types may affect the migration of infected cells away from this transition zone. Future molecular studies will certainly refine our understanding of the precise mechanisms involved. Although this review has concentrated on the functions of the E6 and E7 proteins, it is worth bearing in mind that the expression of other viral non-structural proteins and any interactions they make with E6 and E7 and their association with cellular proteins is likely to have a bearing on function and any precise definition of oncogenic potential. OPEN QUESTIONS AND FUTURE CHALLENGES Far more basic and clinical research has focused on the high-risk HPV types than on the low-risk types despite the substantial financial burden imposed by low-risk HPV types on the health systems of many western countries that have good working cervical cancer screening systems in operation (4), and it is to be hoped that despite the initiation vaccination programmes that also include a tetravalent HPV vaccine that targets HPV types 6 and 11 as well as 16 and 18, investment in research into the biology of low-risk HPV types will be seen as more essential. One significant aspect of our current understanding of the HPV life cycle is that infections, whether by high- or low-risk types, that are able to be cleared by the host immune system in a short period pose much less of a threat in terms of malignancy. In such cases, defects in the host immune response may be a contributory factor and therefore, probably the most challenging future strategy, at least in terms of developing new therapeutic strategies, will be to acquire a better understanding of the molecular interactions that are involved in the way these viruses interface with host immune systems 2010 The Authors Journal Compilation 2010 APMIS HIGH- AND LOW-RISK HPV TARGETS and how the genetic background of the host may affect the outcome of infection. The authors acknowledge the support from the Associazione Italiana per la Ricerca sul Cancro and the International Agency for Research on Cancer and thank Dr Lutz Gissmann for helpful comments. REFERENCES 1.Zur Hausen H. Papillomavirus infections – a major cause of human cancers. Biochem Biophys Acta 1996;1288:55–78. 2.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol 1999;189:12– 9. 3.Derkay CS, Wiatrak B. Recurrent respiratory papillomatosis: a review. Laryngoscope 2008; 118:1236–47. 4.Hu D, Goldie S. The economic burden of noncervical human papillomavirus disease in the United States. Am J Obstet Gynecol 2008; 198:500. 5.Frazer IH. Interaction of human papillomavirus with the host immune system: a well evolved relationship. Virology 2009;384:410–4. 6.Bonagura VR, Siegal FP, Abramson AL, Santiago-Schwarz F, O’Reilly ME, Shah K, et al. Enriched HLA-DQ3 phenotype and decreased class I major histocompatibility complex antigen expression in recurrent respiratory papillomatosis. Clin Diagn Lab Immunol 1994;1:357– 60. 7.Bonagura VR, Hatam L, DeVoti J, Zeng F, Steinberg BM. Recurrent respiratory papillomatosis: altered CD8(+) T-cell subsets and T(H)1 ⁄ T(H)2 cytokine imbalance. Clin Immunol 1999;93:302–11. 8.Vambutas A, Bonagura VR, Reed EF, Abramson AL, Mullooly V, DeVoti J, et al. Polymorphism of transporter associated with antigen presentation 1 as a potential determinant for severity of disease in recurrent respiratory papillomatosis caused by human papillomavirus types 6 and 11. J Infect Dis 2004;189:871–9. 9.Kreimer AR, Clifford GM, Boyle P, Franceschi S. Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev 2005;14:467–75. 10.Schwarz E, Freese UK, Gissmann L, Mayer W, Roggenbuck B, Stremlau A, et al. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature 1985;314: 111–4. 2010 The Authors Journal Compilation 2010 APMIS 11.Smotkin D, Wettstein FO. Transcription of human papillomavirus type 16 early genes in a cervical cancer and a cancer-derived cell line and identification of the E7 protein. Proc Natl Acad Sci USA 1986;83:4680–4. 12.Banks L, Spence P, Androphy E, Hubbert N, Matlashewski G, Murray A, et al. Identification of human papillomavirus type 18 E6 polypeptide in cells derived from human cervical carcinomas. J Gen Virol 1987;68:1351–9. 13.Butz K, Denk C, Ullmann A, Scheffner M, Hoppe-Seyler F. Induction of apoptosis in human papillomavirus positive cancer cells by peptide aptamers targeting the viral E6 oncoprotein. Proc Natl Acad Sci USA 2000;97: 6693–7. 14.Butz K, Ristriani T, Hengstermann A, Denk C, Scheffner M, Hoppe-Seyler F. SiRNA targeting of the viral E6 oncogene efficiently kills human papillomavirus-positive cancer cells. Oncogene 2003;22:5938–45. 15.Goodwin EC, DiMaio D. Repression of human papillomavirus oncogenes in HeLa cervical carcinoma cells causes the orderly reactivation of dominant tumour suppressor pathways. Proc Natl Acad Sci USA 2000;97:12513–8. 16.Spanos WC, Geiger J, Anderson ME, Harris GF, Bossler AD, Smith RB, et al. Deletion of the PDZ motif of HPV-16 E6 preventing immortalization and anchorage-independent growth in human tonsil epithelial cells. Head Neck 2008; 30:139–47. 17.Tsunokawa Y, Takebe N, Kasamatsu T, Terada M, Sugimura T. Transforming activity of human papillomavirus type 16 DNA sequences in cervical cancer. Proc Natl Acad Sci USA 1986;83: 2200–3. 18.Yasumoto S, Burkhardt AL, Doniger J, DiPaolo J. Human papillomavirus type 16 DNA induces malignant transformation of NIH3T3 cells. J Virol 1986;57:572–7. 19.Matlashewski G, Schneider J, Banks L, Jones N, Murray A, Crawford L. Human papillomavirus type 16 DNA cooperates with activated ras in transforming primary cells. EMBO J 1987;6:1741–6. 20.Phelps WC, Yee CL, Münger K, Howley PM. The human papillomavirus type 16 E7 gene encodes transactivation and transformation functions similar to adenovirus E1a. Cell 1988; 53:539–47. 21.Storey A, Pim D, Murray A, Osborn K, Banks L, Crawford L. Comparison of the in vitro transforming activities of human papillomavirus types. EMBO J 1988;7:1815–20. 22.Bedell M, Jones K, Grossman S, Laimins L. Identification of human papillomavirus type 18 transforming genes in immortalized and primary cells. J Virol 1989;63:1247–55. 485 PIM & BANKS 23.Sedman S, Barbosa M, Vass W, Hubbert N, Hass J, Lowy D, et al. The full-length E6 protein of human papillomavirus type 16 has transforming and transactivating activities and cooperates with E7 to immortalize keratinocytes in culture. J Virol 1991;65:4860–6. 24.Storey A, Banks L. Human papillomavirus type 16 E6 gene cooperates with EJ-ras to immortalize primary mouse cells. Oncogene 1993;8:919– 24. 25.Pim D, Storey A, Thomas M, Massimi P, Banks L. Mutational analysis of HPV-18 E6 identifies domains required for p53 degradation in vitro, abolition of p53 transactivation in vivo and immortalisation of primary BMK cells. Oncogene 1994;9:1869–76. 26.Dürst M, Dzarlieva-Petrusevka R, Boukamp P, Fusenig M, Gissman L. Molecular and cytogenic analysis of immortalised human primary keratinocytes obtained after transfection with human papillomavirus type 16 DNA. Oncogene 1987;1: 251–6. 27.Kaur P, McDougall JK. Characterisation of primary human keratinocytes transformed by human papillomavirus type 18. J Virol 1988;62: 917–24. 28.Schlegel R, Phelps WC, Zhang YL, Barbosa M. Quantitative keratinocyte assay detects two biological activities of human papillomavirus DNA and identifies viral types associated with cervical carcinoma. EMBO J 1988;7:3181–7. 29.Sexton CJ, Proby CM, Banks L, Stables JN, Powell K, Navsaria H, et al. Characterisation of factors involved in human papillomavirus type 16-mediated immortalisation of oral keratinocytes. J Gen Virol 1993;74:755–61. 30.Barbosa MS, Schlegel R. The E6 and E7 genes of HPV-18 are sufficient for inducing two-stage in vitro transformation of human keratinocytes. Oncogene 1989;4:1529–32. 31.Hawley-Nelson P, Vousden K, Hubbert N, Lowy D, Schiller J. HPV-16 E6 and E7 proteins cooperate to immortalize human foreskin keratinocytes. EMBO J 1989;8:3905–10. 32.DiPaolo J, Woodworth C, Popescu MC, Notario V, Doniger J. Induction of human cervical squamous cell carcinoma by sequential transfection with human papillomavirus 16 DNA and viral Harvey ras. Oncogene 1989;4:395–9. 33.Dürst M, Gallahan D, Gilbrt J, Rhim JS. Glucocorticoid enhanced neoplastic transformation of human keratinocytes by human papillomavirus type 16 and an activated ras oncogene. Virology 1989;173:767–71. 34.Hurlin PJ, Kaur P, Smith PP, Peres-Reyes N, Blanton R, McDougall JK. Progression of human papillomavirus type 18-immortalised human keratinocytes to a malignant phenotype. Proc Natl Acad Sci USA 1991;88:570–4. 486 35.Pecoraro G, Morgan D, Defendi V. Differential effects of human papillomavirus type 6, 16 and 18 DNAs on immortalisation and transformation of human cervical epithelial cells. Proc Natl Acad Sci USA 1989;86:563–7. 36.Woodworth CD, Doniger J, DiPaolo JA. Immortalisation of human foreskin keratinocytes by various human papillomavirus DNAs corresponds to their association with cervical carcinoma. J Virol 1989;63:159–64. 37.Griep AE, Herber R, Jeon S, Lohse JK, Dubielzig RR, Lambert PF. Tumorigenicity by human papillomavirus type 16 E6 and E7 in transgenic mice correlates with alterations in epithelial cell growth and differentiation. J Virol 1993;67: 1373–84. 38.Arbeit JM, Munger K, Howley PM, Hanahan D. Neuroepithelial carcinomas in mice transgenic with human papillomavirus type 16 E6 ⁄ E7 ORFs. Am J Pathol 1993;142:1187–97. 39.Lambert PF, Pan H, Pitot HC, Liem A, Jackson M, Griep AE. Epidermal cancer associated with expression of human papillomavirus type 16 E6 and E7 oncogenes in the skin of transgenic mice. Proc Natl Acad Sci USA 1993;90:5583–7. 40.Arbeit JM, Münger K, Howley PM, Hanahan D. Progressive squamous epithelial neoplasia in K14-human papillomavirus type 16 transgenic mice. J Virol 1994;68:4358–68. 41.Arbeit JM, Howley PM, Hanahan D. Chronic estrogen-induced cervical and vaginal squamous carcinogenesis in human papillomavirus type 16 transgenic mice. Proc Natl Acad Sci USA 1996;93:2930–5. 42.Chung SH, Lambert PF. Prevention and treatment of cervical cancer in mice using estrogen receptor antagonists. Proc Natl Acad Sci USA 2009;106:19467–72. 43.Song S, Liem A, Miller JA, Lambert PF. Human papillomavirus types 16 E6 and E7 contribute differently to carcinogenesis. Virology 2000;267: 141–50. 44.Shai A, Brake T, Somoza C, Lambert PF. The human papillomavirus E6 oncogene dysregulates the cell cycle and contributes to cervical carcinogenesis through two independent activities. Cancer Res 2007;67:1626–35. 45.Strati K, Lambert PF. Role of Rb-dependent functions of papillomavirus E7 oncogene in head and neck cancer. Cancer Res 2007;67:11585–93. 46.Balsitis S, Dick F, Dyson N, Lambert PF. Critical roles for non-pRb targets of human papillomavirus 16 E7 in cervical carcinogenesis. Cancer Res 2006;66:9393–400. 47.Shin MK, Balsatis S, Brake T, Lambert PF. Human papillomavirus E7 oncoprotein overrides the tumor suppressor activity of p21Cip1 in cervical carcinogenesis. Cancer Res 2009;69: 5656–63. 2010 The Authors Journal Compilation 2010 APMIS HIGH- AND LOW-RISK HPV TARGETS 48.Shai A, Nguyen ML, Wagstaff J, Jiang YH, Lambert PF. HPV16 E6 confers p53-dependent and p53-independent phenotypes in the epidermis of mice deficient for E6AP. Oncogene 2007;26:3321–8. 49.Nguyen ML, Nguyen MM, Lee D, Griep AE, Lambert PF. The PDZ ligand domain of the human papillomavirus type 16 E6 protein is required for E6’s induction of epithelial hyperplasia in vivo. J Virol 2003;77: 6957–64. 50.Blanton RA, Peres-Reyes N, Merrick DT, McDougal JK. Epithelial cells immortalized by human papillomaviruses have premalignant characteristics in organotypic culture. Am J Pathol 1991;138:673–8. 51.Meyers C, Frattini MG, Hudson JB, Laimins LA. Biosynthesis of human papillomavirus from a continuous cell line upon epithelial differentiation. Science 1992;257:971–3. 52.Hummel M, Hudson JB, Laimins LA. Differentiation-induced and constitutive transcription of human papillomavirus type 31b in cell lines containing viral episomes. J Virol 1992;66:6070– 80. 53.Meyers C, Mayer TJ, Ozbun MA. Synthesis of infectious human papillomavirus type 18 in differentiating epithelium transfected with viral DNA. J Virol 1997;71:7381–6. 54.Flores ER, Allen-Hoffmann BL, Lee D, Sattler CA, Lambert PF. Establishment of the human papillomavirus type 16 (HPV-16) life cycle in an immortalized human foreskin keratinocyte line. Virology 1999;262:344–54. 55.Aaltonen LM, Wahlström T, Rihkanen H, Vaheri A. A novel method to culture laryngeal human papillomavirus-positive epithelial cells produces papilloma-type cytology on collagen rafts. Eur J Cancer 1998;34:1111–6. 56.Thomas JT, Oh ST, Terhune SS, Laimins LA. Cellular changes induced by low-risk human papillomavirus type 11 in keratinocytes that stably maintain viral episomes. J Virol 2001;75: 7564–71. 57.Garner-Hamrick PA, Fostel JK, Chien WM, Banerjee NS, Chow LT, Broker TR, et al. Global effects of human papillomavirus type 18 E6 ⁄ E7 in an organotypic keratinocyte culture system. J Virol 2004;78:9041–50. 58.Fang L, Meyers C, Budgeon LR, Howett MK. Induction of productive human papillomavirus type 11 life cycle in epithelial cells grown in organotypic raft cultures. Virology 2006;347:28– 35. 59.Chow LT, Duffy AA, Wang HK, Broker TR. A highly efficient system to produce infectious human papillomavirus: elucidation of natural virus-host interactions. Cell Cycle 2009;8:1319– 23. 2010 The Authors Journal Compilation 2010 APMIS 60.Wang HK, Duffy AA, Broker TR, Chow LT. Robust production and passaging of infectious HPV in squamous epithelium of primary human keratinocytes. Genes Dev 2009;23:181–94. 61.Juntilla MR, Evan GI. P53 – a jack of all trades but master of none. Nat Rev Cancer 2009;11: 821–9. 62.Longworth MS, Dyson NJ. pRB a local chromatin organizer with global possibilities. Chromosoma 2009;119:1–11. 63.Poznic M. Retinoblastoma protein: a central processing unit. J Biosci 2009;34:305–12. 64.Dyson N, Howley PM, Münger K, Harlow E. The human papillomavirus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 1989;243:934–6. 65.Münger K, Werness BA, Dyson N, Phelps WC, Harlow E, Howley PM. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumour suppressor gene product. EMBO J 1989;8:4099–105. 66.Gage J, Meyers C, Wettstein F. The E7 proteins of the nononcogenic human papillomavirus type 6b (HPV-6b) and of the oncogenic HPV-16 differ in retinoblastoma binding and other properties. J Virol 1990;64:723–30. 67.Boyer SN, Wazer DE, Band V. E7 protein of human papilloma virus-16 induces degradation of retinoblastoma protein through the ubiquitinproteasome pathway. Cancer Res 1996;56:4620– 4. 68.Oh KJ, Kalinina A, Bagchi S. Destabilisation of Rb by human papillomavirus E7 is cell cycle dependent: E2-25K is involved in the proteolysis. Virology 2010;396:118–24. 69.Huh K, Zhou H, Hatakawa H, Cho JY, Libermann TA, Jin J, et al. Human papillomavirus type 16 E7 oncoprotein associates with the cullin 2 ubiquitin ligase complex, which contributes to degradation of the retinoblastoma tumour suppressor. J Virol 2007;81:9737–47. 70.Jones DL, Alani RM, Munger K. The human papillomavirus E7 oncoprotein can uncouple cellular differentiation and proliferation in human keratinocytes by abrogating p21cip1-mediated inhibition of cdk2. Genes Dev 1997;11: 2101–11. 71.Helt AM, Galloway DA. Destabilization of the retinoblastoma tumor suppressor by human papillomavirus type 16 E7 is not sufficient to overcome cell cycle arrest in human keratinocytes. J Virol 2001;75:6737–47. 72.Gonzalez SL, Stremlau M, He X, Basile JR, Münger K. Degradation of the retinoblastoma tumour suppressor by the human papillomavirus type 16 E7 oncoprotein is important for functional inactivation and is separable from proteasomal degradation of E7. J Virol 2001;75: 7583–91. 487 PIM & BANKS 73.Zhang B, Chen W, Roman A. The E7 proteins of low- and high-risk human papillomaviruses share the ability to target the pRB family member p130 for degradation. Proc Natl Acad Sci USA 2006;103:437–42. 74.Imai Y, Matsushima Y, Sugimura T, Terada M. Purification and characterization of human papillomavirus type 16 E7 protein with preferential binding to the underphosphorylated form of retinoblastoma gene product. J Virol 1991;65: 4966–72. 75.Hu Q, Dyson N, Harlow E. The regions of retinoblastoma protein needed for binding to adenovirus E1A or SV40 large T antigen are common sites for mutations. EMBO J 1990;9: 1147–55. 76.Kaelin W, Ewen M, Livingston D. Definition of the minimal simian virus 40 large T antigen and adenovirus E1A-binding domain in the retinoblastoma gene product. Mol Cell Biol 1990;10:3761–9. 77.Demers GW, Foster SA, Halbert CL, Galloway DA. Growth arrest by induction of p53 in DNA damaged keratinocytes is bypassed by human papillomavirus 16E7. Proc Natl Acad Sci USA 1994;91:4382–6. 78.Wazer DE, Liu XL, Chu Q, Gao Q, Band V. Immortalisation of distinct human mammary epithelial cell types by human papillomavirus 16 E6 or E7. Proc Natl Acad Sci USA 1995;92: 3687–91. 79.Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature 2001;411: 342–8. 80.Ghittoni R, Accardi R, Hasan U, Gheit T, Sylla B, Tommasino M. The biological properties of E6 and E7 oncoproteins from human papillomaviruses. Virus Genes 2010;40:1–13. 81.McLaughlin-Drubin ME, Münger K. The human papillomavirus E7 oncoprotein. Virology 2009;384:335–44. 82.Edmonds C, Vousden KH. A point mutational analysis of human papillomavirus type 16 E7 protein. J Virol 1989;63:2650–6. 83.Banks L, Edmonds C, Vousden KH. Ability of the HPV16 E7 protein to bind RB and induce DNA synthesis is not sufficient for efficient transforming activity in NIH3T3 cells. Oncogene 1990;5:1383–9. 84.Brehm A, Nielsen SJ, Miska EA, McCance DJ, Reid JL, Bannister AJ, et al. The E7 oncoprotein associates with Mi2 and histone deacetylase activity to promote cell growth. EMBO J 1999; 18:2449–58. 85.Nguyen DX, Westbrook TF, McCance DJ. Human papillomavirus type 16 E7 maintains elevated levels of the cdc25A tyrosine phosphatase during deregulation of cell cycle arrest. J Virol 2002;76:619–32. 488 86.Park JS, Kim EJ, Kwon HJ, Hwang ES, Namkoong SE, Um SJ. Inactivation of interferon regulatory factor-1 tumour suppressor protein by HPV E7 oncoprotein. Implication for the E7mediated immune evasion mechanism in cervical carcinogenesis. J Biol Chem 2000;275:6764–9. 87.Missero C, Calautti E, Eckner R, Chin J, Tsai LH, Livingstone DM, et al. Involvement of the cell-cycle inhibitor Cip1 ⁄ WAF1 and the E1Aassociated protein p300 in terminal differentiation. Proc Natl Acad Sci USA 1995;92:5451–5. 88.Alani RM, Hasskarl J, Münger K. Alterations in cyclin-dependent kinase 2 function during differentiation of human keratinocytes. Mol Carcinog 1998;23:226–33. 89.Martinez LA, Chen Y, Fischer SM, Conti CJ. Coordinated changes in cell cycle machinery occur during keratinocyte terminal differentiation. Oncogene 1999;18:397–406. 90.Funk JO, Waga S, Harry JB, Espling E, Stillman B, Galloway DA. Inhibition of CDK activity and PCNA-dependent DNA replication by p21 is blocked by interaction with the HPV-16 E7 oncoprotein. Genes Dev 1997;11:2090–100. 91.Jones DL, Münger K. Analysis of the p53-mediated G1 growth arrest pathways in cells expressing the human papillomavirus type 16 E7 oncoprotein. J Virol 1997;71:2905–12. 92.Zerfass-Thome K, Zwerschke W, Mannhardt B, Tindle R, Botz JW, Jansen-Dürr P. Inactivation of the cdk inhibitor p27KIP1 by the human papillomavirus type 16 E7 0ncoprotein. Oncogene 1996;13:2323–30. 93.Westbrook TF, Nguyen DX, Thrash BR, McCance D. E7 abolishes raf-induced growth arrest via mislocalisation of p21cip1. Mol Cell Biol 1992;22:7041–52. 94.Pim D, Massimi P, Dilworth SM, Banks L. Activation of the protein B pathway by the HPV-16 E7 oncoprotein occurs through a mechanism involving interaction with PP2A. Oncogene 1995;24:7830–8. 95.Huh KW, DeMasi J, Ogawa H, Nakatani Y, Howley PM, Münger K. Association of the human papillomavirus type 16 E7 oncoprotein with the 600-kDa retinoblastoma protein-associated factor, p600. Proc Natl Acad Sci USA 2005;102:11492–7. 96.Avvakumov N, Torchia J, Mymryk JS. Interaction of the HPV E7 proteins with the pCAF acetyltransferase. Oncogene 2003;22:3833–41. 97.Bernat A, Avvakumov N, Mymryk JS, Banks L. Interaction between the HPV E7 oncoprotein and the transcriptional coactivator p300. Oncogene 2003;22:7871–81. 98.Luscher-Firzlaff JM, Westendorf JM, Zwicker J, Burkhardt H, Henriksson M, Muller R, et al. Interaction of the fork head domain transcription factor MPP2 with the human papillomavirus 2010 The Authors Journal Compilation 2010 APMIS HIGH- AND LOW-RISK HPV TARGETS type 16 E7 protein: enhancement of transformation and transactivation. Oncogene 1999;18: 5620–30. 99.Antinore MJ, Birrer MJ, Patel D, Nader L, McCance DJ. The human papillomavirus type 16 E7 gene product interacts with and trans-activates the AP1 family of transcription factors. EMBO J 1996;15:1950–60. 100.Mazzarelli JM, Atkins GB, Geisberg JV, Ricciardi RP. The viral oncoproteins Ad5 E1A, HPV16 E7 and SV40 TAg bind a common region of the TBP-associated factor-110. Oncogene 1995; 11:859–64. 101.Massimi P, Pim D, Storey A, Banks L. HPV-16 E7 and adenovirus E1A complex formation with TATA box binding protein is enhanced by casein kinase II phosphorylation. Oncogene 1996;12:2325–30. 102.Mannhardt B, Weinzimer SA, Wagner M, Fiedler M, Cohen P, Jansen-Durr P, et al. Human papillomavirus type 16 E7 oncoprotein binds and inactivates growth-inhibitory insulin-like growth factor binding protein 3. Mol Cell Biol 2000;20: 6483–95. 103.Santer FR, Moser B, Spoden GA, Jansen-Durr P, Zwerschke W. Human papillomavirus type 16 E7 oncoprotein inhiits apoptosis mediated by nuclear insulin-like growth factor-binding protein-3 by enhancing its ubiquitin ⁄ proteasome-dependent degradation. Carcinogenesis 2007;28:2511–20. 104.Eichten A, Rud DS, Grace M, Piboonniyom SO, Zacny V, Münger K. Molecular pathways executing the ‘‘trophic sentinel’’ response in HPV-16 E7-expressing normal human diploid fibroblasts upon growth factor depletion. Virology 2004; 319:81–93. 105.Duensing A, Spardy N, Chatterjee P, Zheng L, Parry J, Cuevas R, et al. Centrosome overduplication, chromosomal instability, and human papillomavirus oncoproteins. Environ Mol Mutagen 2009;50:741–7. 106.Hashida T, Yasumoto S. Induction of chromosomal abnormalities in mouse and human epidermal keratinocytes by the human papillomavirus type 16 E7 oncogene. J Gen Virol 1991;72:1569– 77. 107.Fu YS, Reagan JW, Richard RM. Definition of precursors. Gynecol Oncol 1981;12:220–31. 108.White AE, Livanos EM, Tlsty TD. Differential disruption of genomic integrity and cell cycle regulation in normal human fibroblasts by the HPV oncoproteins. Genes Dev 1994;8:666–77. 109.Duensing S, Lee LY, Duensing A, Basile J, Piboonniyom S, Gonzalez S, et al. The human papillomavirus type 16 E6 and E7 oncoproteins cooperate to induce mitotic defects and genomic instability by uncoupling centrosome duplication from the cell division cycle. Proc Natl Acad Sci USA 2000;97:10002–7. 2010 The Authors Journal Compilation 2010 APMIS 110.Duensing S, Duensing A, Flores ER, Do A, Lambert PF, Münger K. Centrosome abnormalities and genomic instability by episomal expression of human papillomavirus type 16 in raft cultures of human keratinocytes. J Virol 2001;75:7712–6. 111.Duensing A, Liu Y, Tseng M, Malumbres M, Barbacid M, Duensing S. Cyclin-dependent kinase 2 is dispensable for normal centrosome duplication but required for oncogene-induced centrosome overduplication. Oncogene 2006;25: 2943–9. 112.Nguyen CL, Eichwald C, Nibert ML, Munger K. Human papillomavirus type 16 E7 oncoprotein associates with the centrosomal component gamma-tubulin. J Virol 2007;81:13533–43. 113.Nguyen CL, McLaughlin-Drubin ME, Münger K. Delocalisation of the microtubule motor dynein from mitotic spindles by the human papillomavirus E7 oncoprotein is not sufficient for induction of multipolar mitoses. Cancer Res 2008;68:8715–22. 114.Nguyen CL, Münger K. Human papillomavirus E7 protein deregulates mitosis via an association with nuclear mitotic apparatus protein 1. J Virol 2009;83:1700–7. 115.Duensing S, Münger K. The human papillomavirus type 16 E6 and E7 oncoproteins independently induce numerical and structural chromosomal instability. Cancer Res 2002;62:7075–82. 116.Gammoh N, Isaacson E, Tomaić V, Jackson DJ, Doorbar J, Banks L. Inhibition of HPV-16 E7 oncogenic activity by HPV-16 E2. Oncogene 2009;28:2299–304. 117.Kessis TD, Connolly DC, Hedrick L, Cho KR. Expression of HPV16 E6 or E7 increases integration of foreign DNA. Oncogene 1996;13: 427–31. 118.Moody CA, Laimins LA. Human papillomaviruses activate the ATM DNA damage pathway for viral genome amplification upon differentiation. PLoS Pathog 2009;5:e1000605. 119.Chen B, Simpson DA, Zhou Y, Mitra A, Mitchell DL, Cordeiro-Stone M, et al. Human papillomavirus type 16 E6 deregulates CHK1 and sensitizes human fibroblasts to environmental carcinogens independently of its effects on p53. Cell Cycle 2009;8:1775–87. 120.Werness B, Levine A, Howley P. Association of human papillomavirus types 16 and 18 E6 proteins with p53. Science 1990;248:76–9. 121.Scheffner M, Werness BA, Huibregtse JM, Levine AJ, Howley PM. The E6 oncoprotein encoded by human papillomavirus types 16 and 18 promotes the degradation of p53. Cell 1990;63:1129–36. 122.Haupt Y, Maya R, Kazaz A, Oren M. Mdm2 promotes the rapid degradation of p53. Nature 1997;387:296–9. 489 PIM & BANKS 123.Kubbutat MH, Jones SN, Vousden KH. Regulation of p53 stability by Mdm2. Nature 1997;387: 299–303. 124.Huibregtse J, Scheffner M, Howley P. A cellular protein mediates the association of p53 with the E6 oncoprotein of human papillomavirus types 16 or 18. EMBO J 1991;10:4129–35. 125.Scheffner M, Huibregtse J, Vierstra R, Howley P. The HPV-16 E6 and E6-AP complex functions as a ubiquitin ligase in the ubiquitination of p53. Cell 1993;75:495–505. 126.Crook T, Tidy JA, Vousden KH. Degradation of p53 can be targeted by HPV E6 sequences distinct from those required for p53 binding and trans-activation. Cell 1991;67:547–56. 127.Lechner MS, Laimins LA. Inhibition of p53 DNA binding by human papillomavirus E6 proteins. J Virol 1994;68:4262–73. 128.Lechner M, Mack D, Finicle A, Crook T, Vousden K, Laimins L. Human papillomavirus E6 proteins bind p53 in vivo and abrogate p53-mediated repression of transcription. EMBO J 1992;11:3045–52. 129.Mietz J, Unger T, Huibregtse J, Howley P. The transcriptional transactivation function of wild type p53 is inhibited by SV40 large T-antigen and by HPV-16 E6 oncoprotein. EMBO J 1992;11: 5013–20. 130.Li X, Coffino P. High risk human papillomavirus E6 protein has two distinct binding sites within p53, of which only one determines degradation. J Virol 1996;70:4509–16. 131.Sun L, Zhang G, Lei T, Huang C, Song T, Si L. Two different HPV-11E6 fusion proteins trap p53 in the cytoplasm and induce apoptosis. Cancer Biol Ther 2008;7:1909–15. 132.Stewart D, Ghosh A, Matlasheski G. Involvement of nuclear export in human papillomavirus type 18 E6-mediated ubiquitination and degradation of p53. J Virol 2005;79:8773–83. 133.Shamanin VA, Sekaric P, Androphy EJ. hAda3 degradation by papillomavirus type 16 E6 correlates with abrogation of the p14ARF-p53 pathway and efficient immortalisation of human mammary epithelial cells. J Virol 2008;82:3912– 20. 134.Hu Y, Ye F, Lu W, Hong D, Wan X, Xie X. HPV16 E6-induced and E6AP-dependent inhibition of the transcriptional coactivator hADA3 in human cervical carcinoma cells. Cancer Invest 2009;27:298–306. 135.Thomas MC, Chiang CM. E6 oncoprotein represses p53-dependent gene activation via inhibition of protein acetylation independently of inducing p53 degradation. Mol Cell 2005;17:251– 64. 136.Ishiwatari H, Hayasaka N, Inoue H, Yutsudo M, Hakura A. Degradation of p53 only is not sufficient for the growth stimulatory effect of human 490 papillomavirus 16 E6 oncoprotein in human embryonic fibroblasts. J Med Virol 1994;44: 243–9. 137.Nakagawa S, Watanabe S, Yoshikawa H, Taketani Y, Yoshiike K, Kanda T. Mutational analysis of human papillomavirus type 16 E6 protein: transforming function for human cells and degradation of p53 in vitro. Virology 1995;212:535–42. 138.Thomas M, Banks L. Inhibition of Bak-induced apoptosis by HPV-18 E6. Oncogene 1998;17: 2943–54. 139.Thomas M, Banks L. Human papillomavirus (HPV) E6 interactions with Bak are conserved amongst E6 proteins from high and low risk HPV types. J Gen Virol 1999;80:1513–7. 140.Jackson S, Harwood C, Thomas M, Banks L, Storey A. Role of Bak in UV-induced apoptosis in skin cancer and abrogation by HPV E6 proteins. Genes Dev 2000;14:3065–73. 141.Zimmermann H, Degenkolbe R, Bernard HU, O’Connor MJ. The human papillomavirus type 16 E6 oncoprotein can down-regulate p53 activity by targeting the transcriptional coactivator CBP ⁄ p300. J Virol 1999;73:6209–19. 142.Patel D, Huang SM, Baglia LA, McCance DJ. The E6 protein of human papillomavirus type 16 binds to and inhibits co-activation by CBP and p300. EMBO J 1999;18:5061–72. 143.Chen JJ, Reid C, Band V, Androphy EJ. Interaction of papillomavirus E6 oncoproteins with a putative calcium-binding protein. Science 1995;269:529–31. 144.Kühne C, Banks L. E3-ubiquitin ligase ⁄ E6-AP links multicopy maintenance protein 7 to the ubiquitination pathway by a novel motif, the L2G box. J Biol Chem 1998;273:34302–9. 145.Gross-Mesilaty S, Reinstein E, Bercovich B, Tobias KE, Schwartz AL, Kahana C, et al. Basal and human papillomavirus E6 oncoproteininduced degradation of Myc proteins by the ubiquitin pathway. Proc Natl Acad Sci USA 1998;95:8058–63. 146.Tong X, Howley P. The bovine papillomavirus E6 oncoprotein interacts with paxillin and disrupts the actin cytoskeleton. Proc Natl Acad Sci USA 1997;94:4412–7. 147.Wade R, Brimer N, Vande Pol S. Transformation by bovine papillomavirus type 1 E6 requires paxillin. J Virol 2008;82:5962–6. 148.McCormack SJ, Brazinski SE, Moore JL Jr, Werness BA, Goldstein DJ. Activation of the focal adhesion kinase signal transduction pathway in cervical carcinoma cell lines and human genital epithelial cells immortalized with human papillomavirus type 18. Oncogene 1997;15:265– 74. 149.Li S, Labrecque S, Gauzzi MC, Cuddihy AR, Wong AHT, Pellegrini S, et al. The human papilloma virus (HPV)-18 E6 oncoprotein physically 2010 The Authors Journal Compilation 2010 APMIS HIGH- AND LOW-RISK HPV TARGETS interacts with Tyk2 and impairs Jak-STAT activation by interferon-a. Oncogene 1999;18:5727– 37. 150.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res 1965;37: 614–36. 151.Greider CW, Blackburn EH. Identification of a specific telomere transferase activity in Tetrahymena extracts. Cell 1985;43:405–13. 152.Artandi SE, DePinho RA. Telomeres and telomerase in cancer. Carcinogenesis 2010;31:9–18. 153.Klingelhutz AJ, Barber SA, Smith PP, Dyer K, McDougall JK. Restoration of telomeres in human papillomavirus-immortalized human anogenital epithelial cells. Mol Cell Biol 1994;14: 961–9. 154.Klingelhutz AJ, Foster SA, McDougall JK. Telomerase activation by the E6 gene product of human papillomavirus type 16. Nature 1996;380: 79–82. 155.Liu Y, Chen JJ, Gao Q, Dalal S, Hong Y, Mansur CP, et al. Multiple functions of human papillomavirus type 16 E6 contribute to the immortalisation of mammary epithelial cells. J Virol 1999;73:7297–307. 156.James MA, Lee JH, Klingelhutz AJ. HPV-16 E6 associated hTERT promoter acetylation is E^AP dependent, increased in later passage cells and enhanced by loss of p300. Int J Cancer 2006;119: 1878–85. 157.Oh ST, Kyo S, Laimins LA. Telomerase activation by papillomavirus type 16 E6 protein: induction of human telomerase reverse transcriptase expression through Myc and GC-rich Sp1 binding sites. J Virol 2001;75:5559–66. 158.Veldman T, Liu X, Yuan H, Schlegel R. Human papillomavirus E6 and Myc proteins associate in vivo and bind to and cooperatively activate the telomerase reverse transcriptase promoter. Proc Natl Acad Sci USA 2003;100:8211–6. 159.Liu X, Dakic A, Zhang Y, Dai Y, Chen R, Schlegel R. HPV E6 protein interacts physically and functionally with the cellular telomerase complex. Proc Natl Acad Sci USA 2009;106: 18780–5. 160.Liu X, Yuan H, Fu B, Disbrow GL, Apolinario T, Tomaić V, et al. The E6AP ubiquitin ligase is required for transactivation of the hTERT promoter by the human papillomavirus E6 oncoprotein. J Biol Chem 2005;280:10807–16. 161.Gewin L, Myers H, Galloway DA. Identification of a novel telomerase repressor that interacts with human papillomavirus type-16 E6 ⁄ E6-AP complex. Genes Dev 2004;18:2269–82. 162.Sekaric P, Cherry JJ, Androphy EJ. Binding of the human papillomavirus type 16 E6 to E6AP is not required for activation of hTERT. J Virol 2008;82:71–6. 2010 The Authors Journal Compilation 2010 APMIS 163.Liu X, Roberts J, Dakic A, Zhang Y, Schlegel R. HPV E7 contributes to the telomerase activity of immortalized and tumorigenic cells and augments E6-induced hTERT promoter function. Virology 2008;375:611–23. 164.Deng Z, Lezina L, Chen CJ, Shtivelband S, So W, Lieberman PM. Telomeric proteins regulate episomal maintenance of Epstein–Barr virus origin of replication. Mol Cell 2002;9:493–503. 165.Fanning AS, Anderson JM. PDZ domains: fundamental building blocks in the organization of protein complexes at the plasma membrane. J Clin Invest 1999;103:767–72. 166.Pim D, Thomas M, Javier R, Gardiol D, Banks L. HPV E6 targeted degradation of the discs large protein: evidence for the involvement of a novel ubiquitin ligase. Oncogene 2000;19:719–25. 167.Thomas M, Glaunsinger B, Pim D, Javier R, Banks L. HPV E6 and MAGUK protein interactions: determination of the molecular basis for specific protein recognition and degradation. Oncogene 2001;20:5431–9. 168.Lee S, Glaunsinger B, Mantovani F, Banks L, Javier R. The multi-PDZ domain protein MUPP1 is a cellular target for both human adenovirus E4-ORF1 and high-risk papillomavirus type 18 E6 oncoproteins. J Virol 2000;74:9680– 93. 169.Thomas M, Laura R, Hepner K, Guccione E, Sawyers C, Lasky L, et al. Oncogenic human papillomavirus E6 proteins target the MAGI-2 and MAGI-3 proteins for degradation. Oncogene 2002;21:5088–96. 170.Thomas M, Massimi P, Navarro C, Borg JP, Banks L. The hScrib ⁄ Dlg apico-basal control complex is differentially targeted by HPV-16 and HPV-18 E6 proteins. Oncogene 2005;24:6222–30. 171.Gardiol D, Galizzi S, Banks L. Mutational analysis of the discs large tumour suppressor identifies domains responsible for HPV-18 E6 mediated degradation. J Gen Virol 2002;83:283– 9. 172.Kiyono T, Hiraiwa A, Fujita M, Hayashi Y, Akiyama T, Ishibashi M. Binding of high-risk human papillomavirus E6 oncoproteins to a human homologue of the Drosophila discs large tumour suppressor protein. Proc Natl Acad Sci USA 1997;94:11612–6. 173.Gardiol D, Kühne C, Glaunsinger B, Lee SS, Javier R, Banks L. Oncogenic human papillomavirus E6 proteins target the discs large tumour suppressor for proteasome-mediated degradation. Oncogene 1999;18:5487–96. 174.Nakagawa S, Huibregtse JM. Human scribble (vartul) is targeted for ubiquitin-mediated degradation by the high-risk papillomavirus E6 proteins and the E6AP ubiquitin ligase. Mol Cell Biol 2000;20:8244–53. 491 PIM & BANKS 175.Glaunsinger BA, Lee SS, Thomas M, Banks L, Javier R. Interaction of the PDZ-protein MAGI1 with adenovirus E4-ORF1 and high-risk papillomavirus E6 oncoproteins. Oncogene 2000;19:5270–80. 176.Thomas M, Narayan N, Pim D, Tomaić V, Massimi P, Nagasaka K, et al. Human papillomaviruses, cervical cancer and cell polarity. Oncogene 2008;27:7018–30. 177.Massimi P, Gammoh N, Thomas M, Banks L. HPV E6 specifically targets different cellular pools of its PDZ domain-containing tumour suppressor substrates for proteasome-mediated degradation. Oncogene 2004;23:8033–9. 178.Narayan N, Massimi P, Banks L. CKD phosphorylation of the discs large tumour suppressor controls its localization and stability. J Cell Sci 2009;122:65–74. 179.Narayan N, Subbaiah VK, Banks L. The highrisk E6 oncoprotein preferentially targets phosphorylated nuclear forms of hDlg. Virology 2009;387:1–4. 180.Humbert P, Russell S, Richardson H. Dlg, Scribble and Lgl in cell polarity, cell proliferation and cancer. Bioessays 2003;25:542–53. 181.Shin K, Straight S, Margolis B. PATJ regulates tight junction formation and polarity in mammalian epithelial cells. J Cell Biol 2005;168: 705–11. 182.Hamazaki Y, Itoh M, Sasaki H, Furuse M, Tsukita S. Multi-PDZ domain protein 1 (MUPP1) is concentrated at tight junctions through its possible interaction with claudin-1 and junctional adhesion molecule. J Biol Chem 2002;277:455–61. 183.Dobrosotskaya IY, James GL. MAGI-1 interacts with beta-catenin and is associated with cell-cell adhesion structures. Biochem Biophys Res Commun 2000;270:903–9. 184.Wu X, Hepner K, Castelino-Prabhu S, Do D, Kaye MB, Yuan X-J, et al. Evidence for regulation of the PTEN tumour suppressor by a membrane-localized multi-PDZ domain containing scaffold protein MAGI-2. Proc Natl Acad Sci USA 2000;97:4233–8. 185.Tomaić V, Gardiol D, Massimi P, Ozbun M, Myers M, Banks L. Human and primate tumour viruses use PDZ binding as an evolutionarily conserved mechanism of targeting cell polarity regulators. Oncogene 2009;28:1–8. 186.Lee C, Laimins LA. Role of the PDZ domainbinding motif of the oncoprotein E6 in the pathogenesis of human papillomavirus type 31. J Virol 2004;74:12366–77. 187.Jing M, Bohl J, Brimer N, Kinter m, vande Pol SB. Degradation of tyrosine phosphatase PTPN3 (PTPH1) by association with oncogenic human papillomavirus E6 proteins. J Virol 2007;81: 2231–9. 492 188.Töppfer S, Müller-Schiffmann A, Matentzoglu K, Scheffner M, Steger G. Protein tyrosine phosphatase H1 is a target of the E6 oncoprotein of high-risk genital human papillomaviruses. J Gen Virol 2007;88:2956–65. 189.Spanos WC, Hoover A, Harris GF, Wu S, Strand GL, Anderson ME, et al. The PDZ binding motif of human papillomavirus type 16 E6 induces PTPN13 loss, which allows anchorageindependent growth and synergizes with ras for invasive growth. J Virol 2008;82:2493–500. 190.Hoover AC, Strand GL, Nowicki PN, Anderson ME, Vermeer PD, Klingelhutz AJ, et al. Impaired PTPN13 phosphatase activity in spontaneous or HPV-induced squamous cell carcinomas potentiates oncogene signaling through the MAP kinase pathway. Oncogene 2009;28:3960–70. 191.Pim D, Thomas M, Banks L. Chimaeric HPV E6 proteins allow dissection of the proteolytic pathways regulating different cellular target proteins. Oncogene 2002;21:8140–8. 192.Matlashewski GJ, Tuck S, Pim D, Lamb P, Schneider J, Crawford L. Primary structure polymorphism at amino acid residue 72 of human p53. Mol Cell Biol 1987;7:961–3. 193.Storey A, Thomas M, Kalita A, Harwood C, Gardiol D, Mantovani F, et al. Role of a p53 polymorphism in the development of human papillomavirus-associated cancer. Nature 1998; 393:229–34. 194.Brimer N, Lyons C, Vande Pol SB. Association of E6AP (UBE3A) with human papillomavirus type 11 E6 protein. Virology 2007;358: 303–10. 195.Sterlinko Grm H, Weber M, Elston R, McIntosh P, Griffin H, Banks L, et al. Inhibition of E6induced degradation of its cellular substrates by novel blocking peptides. J Mol Biol 2004;335: 971–85. 196.Sterlinko Grm H, Banks L. Degradation of hDlg and MAGIs by human papillomavirus E6 is E6-AP-independent. J Gen Virol 2004;85: 2815–9. 197.Camus S, Menéndez S, Cheok CF, Stevenson LF, Laı́n S, Lane DP. Ubiquitin-independent degradation of p53 mediated by high-risk human papillomavirus protein E6. Oncogene 2007;26:4059–70. 198.Massimi P, Shai A, Lambert P, Banks L. HPV E6 degradation of p53 and PDZ containing substrates in an E6AP null background. Oncogene 2008;27:1800–4. 199.Tomaić V, Pim D, Banks L. The stability of the human papillomavirus E6 oncoprotein is E6AP dependent. Virology 2009;393:7–10. 200.Vos RM, Altreuter J, White EA, Howley PM. The ubiquitin-specific peptidase USP15 regulates human papillomavirus type 16 E6 protein stability. J Virol 2009;83:8885–92. 2010 The Authors Journal Compilation 2010 APMIS HIGH- AND LOW-RISK HPV TARGETS 201.Doorbar J, Parton A, Hartley K, Banks L, Crook T, Stanley M, et al. Detection of novel splicing patterns in a HPV16-containing keratinocyte cell line. Virology 1990;178:254–62. 202.Pim D, Tomaić V, Banks L. The HPV E6* proteins from high-risk, mucosal human papillomaviruses can direct the degradation of cellular proteins in the absence of full-length E6 protein. J Virol 2009;393:7–10. 203.Schneider-Gädicke A, Schwarz E. Different human cervical carcinoma cell lines show similar transcription patterns of human papillomavirus type 18 early genes. EMBO J 1986;5: 2285–92. 204.Pim D, Massimi P, Banks L. Alternatively spliced HPV-18E6* protein inhibits E6 mediated degradation of p53 and suppresses transformed cell growth. Oncogene 1997;15:257–64. 205.Pim D, Banks L. HPV-18 E6*I protein modulates the E6-mediated degradation of p53 by binding to full-length HPV-18 E6. Oncogene 1999;18:7403–8. 2010 The Authors Journal Compilation 2010 APMIS 206.Filippova M, Johnson MM, Bautista M, Filippov V, Fodor N, Tungteakkhun SS, et al. The large and small isoforms of human papillomavirus type 16 E6 bind to and differentially affect procaspase 8 stability and activity. J Virol 2007;81:4116–29. 207.Storrs CH, Silverstein SJ. PATJ, a tight junctionassociated PDZ protein, is a novel degradation target of high-risk human papillomavirus E6 and the alternatively spliced isoform 18 E6*. J Virol 2007;81:4080–90. 208.Peh WL, Middleton K, Christensen N, Nicholls P, Egawa K, Sotlar K, et al. Life cycle heterogeneity in animal models of human papillomavirus-associated disease. J Virol 2002;76: 10401–16. 209.Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci 2006;110:525–54. 493