Chemistry – Unit 1 Review Name Date Pd 1. What is the difference
advertisement

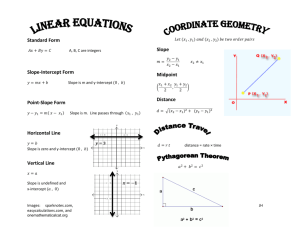
Chemistry – Unit 1 Review 1. Name Date Pd What is the difference between mass, volume and density? What are some of the units we have used for each? 2. State the Law of Conservation of Mass in the words we used in class. Summarize the class results for Parts 1-6 of Lab 1, draw “before” and “after” particle diagrams and briefly explain how each part gave evidence for this law. Law of Conservation of Mass PART Class Results: Change in Mass? 1: ____________ 2: ____________ 3: ____________ 4: ____________ 5: ____________ 6: ____________ 3. If the box at left contains atoms of aluminum in the solid phase, represent the same atoms in the in the gaseous phase in the right hand box. Explain your diagram 4. On the left is a graduated cylinder containing water. An object with mass of 25.0 g and volume of 7.0 mL is placed in the water. Sketch the object and the new water level in the graduated cylinder to the right. Modeling Chemistry 1 U1 review v2.1 5. The chemistry class produced the following graph when they plotted the measured volume of water in mL vs the calculated volume of the container measured in cm3. a. Write a sentence that shows the meaning of the slope value. Start with “For every………….” b. Is the Y-intercept significant? Why or why not? Use the 5% rule. If Y-intercept ‹ 5% Y-max, b=0. If Y-intercept > 5% Y-max, it is significantly NOT zero. c. 6. How could you account for the fact that they obtained a negative y-intercept? The chemistry class produced the following graph when they were measuring the mass and volume of a set of objects in the lab. Modeling Chemistry 2 U1 review v2.1 a. What information about the stubstances is given by the slope of the graph? Show on the graph how slope is calculated. Do not use just one data point to calculate slope! b. Is each the Y-intercept significant? Why or why not? Use the 5% rule. If Y-intercept ‹ 5% Y-max, b=0. If Y-intercept > 5% Y-max, it is significantly NOT zero. c. What would you predict would happen if you were to put each of the objects in water? Explain. d. Use the graph to determine: ➔ the mass of a 7 cm3 piece of the orange substance. Show answer and work on graph. ➔ the volume of a 25 g piece of the silver substance. Show answer and work on graph. e. Draw a particle diagram for each substance for a 20 g sample of each. Explain! your diagrams. f. Draw a particle diagram for each substance for a 8 mL sample of each. Explain! your diagrams. Density Problems: Use proportions with units to complete the following. Show all your work!! 8. Mercury has a density of 13.6g/mL. What is the volume occupied by 112.0 grams of mercury? 9. A cube of gold-colored metal with a volume of 54 cm3 has a mass of 980 g. The density of gold is 19.3 g/cm3. Is this sample of metal pure gold? Why or why not? 10. Metric Conversions: Use proportions to make the following conversions. Show work with units! Modeling Chemistry 3 U1 review v2.1 a. 579.4 mL = ___kL b. 809 mg = ____ g c. 54 km = ____ cm d. 2.67 x 10⁴ g = ___ μg 11. Significant figures: Rounding a. 703.590 cm Round to 4 sig fig Round to 1 sig fig b. 0.04684 g Round to 2 sig figs Round to 1 sig fig 12. Significant Figures: Calculations Calculator answer Rounded answer a. 9.00 cm X 23.46 cm = b. 8.1 + 14.356 – 7.0 = c. 83000/24.65 = d. 7500 - 506.5 = Rule for adding and subtracting: Rule for multiplying and dividing: Lab Application: Modeling Chemistry 4 U1 review v2.1 16. A 1.25 g piece of a substance is rolled out into a thin sheet measuring 14.1 cm X 15.2 cm. The mass of the substance is 2.7 g/cm3. Determine the thickness of the thin sheet. Show all your work with units! Formulas: a. D = M/V V = A X h Calculate the volume of the sheet of metal. b. Calculate the thickness (height) of the sheet of metal. c. How many atoms thick is this thin piece of metal? Diameter of 1 atom = 2.5 x 10-8cm. Modeling Chemistry 5 U1 review v2.1