What is Acid Rain and What Causes It?
advertisement

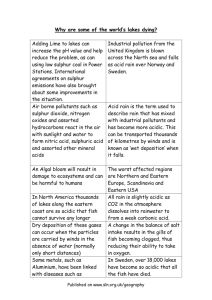
What is Acid Rain and What Causes It? “Acid rain” is a broad term used to describe several ways that acids fall out of the atmosphere. A more precise term is acid deposition, which has two parts: wet and dry. Wet deposition refers to acidic rain, fog, and snow. As this acidic water flows over and through the ground, it affects a variety of plants and animals. The strength of the effects depend on many factors, including how acidic the water is, the chemistry and buffering capacity of the soils involved, and the types of fish, trees, and other living things that rely on the water. Dry deposition refers to acidic gases and particles. About half of the acidity in the atmosphere falls back to Earth through dry deposition. The wind blows these acidic particles and gases onto buildings, cars, homes, and trees. Dry deposited gases and particles can also be washed from trees and other surfaces by rainstorms. When that happens, the runoff water adds those acids to the acid rain, making the combination more acidic than the falling rain alone. Prevailing winds blow the compounds that cause both wet and dry acid deposition across state and national borders, and sometimes over hundreds of miles. Scientists discovered, and have confirmed, that sulfur dioxide (SO2) and nitrogen oxides (NOx) are the primary causes of acid rain. Almost all of SO2 and some NOx come from electric power generation that relies on burning fossil fuels like coal. Acid rain occurs when these gases react in the atmosphere with water, oxygen, and other chemicals to form various acidic compounds. Sunlight increases the rate of most of these reactions. The result is a mild solution of sulfuric acid and nitric acid. Acid rain is measured using a scale called pH. The lower a substance's pH, the more acidic it is. Pure water has a pH of 7.0, which is neutral. Normal rain is slightly acidic because carbon dioxide dissolves into it, so it has a pH of about 5.5. In the year 2000, the most acidic rain falling in North America had a pH of about 4.3. Effects of Acid Rain Acid rain causes acidification of lakes and streams and contributes to damage of trees at high elevations (for example, red spruce trees above 2,000 feet) and many sensitive forest soils. In addition, acid rain accelerates the decay of building materials and paints, including irreplaceable buildings, statues, and sculptures that are part of a nation's cultural heritage. Prior to falling to the Earth, SO2 and NOx gases and their particulate matter derivatives, sulfates and nitrates, contribute to visibility degradation and harm human health. 7 Effects on materials and buildings: Acid rain and the dry deposition of acidic particles contribute to the corrosion of metals (such as bronze) and the deterioration of paint and stone (such as marble and limestone). These effects seriously reduce the value to society of buildings, bridges, cultural objects (such as statues, monuments, and tombstones), and cars. Dry deposition of acidic compounds can also dirty buildings and other structures, leading to increased maintenance costs. Effects on water bodies: The ecological effects of acid rain are most clearly seen in the aquatic or water environments such as streams, lakes, and marshes. Acid rain flows into streams, lakes, and marshes after falling on forests, fields, buildings, and roads. Acid rain also falls directly on aquatic habitats. Most lakes and streams have a pH between 6 and 8, although some lakes are naturally acidic even without the effects of acid rain. Acid rain primarily affects sensitive bodies of water, which are located in watersheds whose soils have a limited ability to neutralize acidic compounds (called "buffering capacity"). Lakes and streams become acidic (pH value goes down) when the water itself and its surrounding soil cannot buffer the acid rain enough to neutralize it. In areas where buffering capacity is low, acid rain also releases aluminum from soils into lakes and streams. Many lakes and streams examined in developed countries suffer from chronic acidity, a condition in which water has a constant low pH level. Streams flowing over soil with low buffering capacity are as susceptible to damage from acid rain as lakes. The acidification problem grows in magnitude if "episodic acidification" is taken into account. Episodic acidification refers to brief periods during which pH levels decrease due to runoff from melting snow or heavy downpours. Lakes and streams in many areas are sensitive to episodic acidification. A lot more lakes and streams become temporarily acidic during storms and spring snowmelt. Acid rain causes a cascade of effects that harm or kill individual fish, reduce fish population numbers, completely eliminate fish species from a water body, and decrease biodiversity. As acid rain flows through soils with low buffering capacity in a watershed, aluminum is released from soils into the lakes and streams located in that watershed. So, as pH in a lake or stream decreases, aluminum levels increase. Both low pH and increased aluminum levels are directly toxic to fish. In addition, low pH and increased aluminum levels cause chronic stress that may not kill individual fish, but leads to lower body weight and smaller size and makes fish less able to compete for food and habitat. Some types of plants and animals are able to tolerate acidic waters. Others, however, are acid-sensitive and will be lost as the pH declines. Generally, the young of most species are more sensitive to environmental conditions than 8 adults. At pH 5, most fish eggs cannot hatch. At lower pH levels, some adult fish die. Some acid lakes have no fish at all. In an ecosystem, different organisms are interdependent on each other. So even if some fish are able to tolerate the acidic waters, their food – consisting of smaller fish or insects – may not survive the increased acidity. Effects on forest floors: A spring shower in the forest washes leaves and falls through the trees to the forest floor below. Some trickles over the ground and runs into a stream, river, or lake, and some of the water soaks into the soil. That soil may neutralize some or all of the acidity of the acid rainwater. This ability is called buffering capacity, and without it, soils become more acidic. Differences in soil buffering capacity are an important reason why some areas that receive acid rain show a lot of damage, while other areas that receive about the same amount of acid rain do not appear to be harmed at all. The ability of forest soils to resist, or buffer, acidity depends on the thickness and composition of the soil, as well as the type of bedrock beneath the forest floor. Effects on plants and trees: Acid rain does not usually kill plants and trees directly. Instead, it is more likely to weaken them by damaging their leaves, limiting the nutrients available to them, or exposing them to toxic substances slowly released from the soil. Quite often, injury or death of trees is a result of these effects of acid rain in combination with one or more additional threats. Scientists know that acidic water dissolves the nutrients and helpful minerals in the soil and then washes them away before trees and other plants can use them to grow. At the same time, acid rain causes the release of substances that are toxic to trees and plants, such as aluminum, into the soil. Scientists believe that this combination of loss of soil nutrients and increase of toxic aluminum may be one way that acid rain harms plants and trees. Such substances also wash away in the runoff and are carried into streams, rivers, and lakes. More of these substances are released from the soil when the rainfall is more acidic. However, trees can be damaged by acid rain even if the soil is well buffered. Forests in high mountain regions often are exposed to greater amounts of acid than other forests because they tend to be surrounded by acidic clouds and fog that are more acidic than rainfall. Scientists believe that when leaves are frequently bathed in this acid fog, essential nutrients in their leaves and needles are stripped away. This loss of nutrients in their foliage makes trees more susceptible to damage by other environmental factors, particularly cold winter weather. Although damaged by other air pollutants such as ground level ozone, food crops are not usually seriously affected because farmers frequently add fertilizers to the soil to replace nutrients that have washed away. They may also add crushed limestone to the soil. Limestone is an alkaline material and increases the ability of the soil to act as a buffer against acidity. 9 Ways of reducing the harmful effects of acid deposition Awareness is being generated among the masses worldwide about acid rain and its harmful effects. This in turn would lead to the politicians being provided with more and better solutions as well as being pressurized to take effective steps to control acid rain. The following are a few steps that could be taken in this regard: Clean up smokestacks and exhaust pipes: Almost all of the electricity that powers modern life comes from burning fossil fuels like coal, natural gas, and oil. Acid deposition is caused by two pollutants that are released into the atmosphere, or emitted, when these fuels are burned: sulfur dioxide (SO2) and nitrogen oxides (NOx). Coal accounts for most for most of the sulfur dioxide (SO2) emissions and a large portion of NOx emissions. Sulfur is present in coal as an impurity, and it reacts with air when the coal is burned to form SO2. In contrast, NOx is formed when any fossil fuel is burned. There are several options for reducing SO2 emissions, including using coal containing less sulfur, washing the coal, and using devices called scrubbers to chemically remove the SO2 from the gases leaving the smokestack. Power plants can also switch fuels; for example burning natural gas creates much less SO2 than burning coal. Certain approaches will also have additional benefits of reducing other pollutants such as mercury and carbon dioxide. Understanding these co-benefits has become important in seeking costeffective air pollution reduction strategies. Finally, power plants can use technologies that don't burn fossil fuels. But each of these options has its own costs and benefits. Similar to scrubbers on power plants, catalytic converters reduce NOx emissions from cars. Use alternative energy sources: There are other sources of electricity besides fossil fuels. They include: nuclear power, hydropower, wind energy, geothermal energy, and solar energy. Of these, nuclear and hydropower are used most widely. Wind, solar, and geothermal energy have not yet been harnessed on a large scale, but are potential alternatives. There are also alternative energies available to power automobiles, including natural gas powered vehicles, battery-powered cars, fuel cells, and combinations of alternative and gasoline powered vehicles. All sources of energy have environmental costs as well as benefits. Some types of energy sources are more expensive to harness than others, which means that not all people can afford all types of energy. Nuclear power, hydropower, and coal are the cheapest forms today, but changes in technologies and environmental regulations may shift that in the future. All of these factors must be weighed when deciding which energy source to use today and which to invest in for tomorrow. 10 Restore a damaged environment: Acid deposition penetrates deeply into the fabric of an ecosystem, changing the chemistry of the soil as well as the chemistry of the streams and narrowing, sometimes to nothing, the space where certain plants and animals can survive. Because there are so many changes, it takes many years for ecosystems to recover from acid deposition, even after emissions are reduced and the rain becomes normal again. For example, while the visibility might improve within days, and small or episodic chemical changes in streams improve within months, chronically acidified lakes, streams, forests, and soils can take years to decades or even centuries (in the case of soils) to heal. However, there are some things that people do to bring back lakes and streams more quickly. Limestone or lime (a naturally-occurring basic compound) can be added to acidic lakes to neutralize the acidity. This process, called liming, has been used extensively in Norway and Sweden but is not used very often in the United States. Liming tends to be expensive, has to be done repeatedly to keep the water from returning to its acidic condition, and is considered a short-term remedy in only specific areas rather than an effort to reduce or prevent pollution. Furthermore, it does not solve the broader problems of changes in soil chemistry and forest health in the watershed, and does nothing to address visibility reductions, materials damage, and risk to human health. However, liming does often permit fish to remain in a lake, so it allows the native population to survive in place until emissions reductions reduce the amount of acid deposition in the area. Take action as individuals: Like many environmental problems, acid deposition is caused by the cumulative actions of millions of individual people. Therefore, each individual can also reduce their contribution to the problem and become part of the solution. So steps are being taken to create awareness among the masses. People are being educated worldwide – especially in the developed countries – regarding that individuals can contribute directly by conserving energy, since energy production causes the largest portion of the acid deposition problem. For example, one can: • Turn off lights, computers, and other electric appliances when they are not being used. • Use energy efficient appliances: lighting, air conditioners, heaters, refrigerators, washing machines, etc. • Insulate the houses as best as possible so as to reduce energy losses. • Use public transportation rather than personal cars, or even walk or bicycle whenever doable. • Buy vehicles with low NOx emissions, and maintain all vehicles well so that they release lesser emissions. Research by Ahmad Raza sahmadraza@yahoo.com Reference contents URLs http://www.epa.gov/airmarkets/acidrain/index.html http://www.policyalmanac.org/environment/archive/acid_rain.shtml 11