Crystal Physics
advertisement

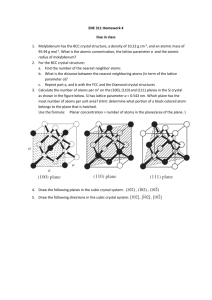
< Engineering Physics -I > < Crystal Physics – Lattice, Unit Cell and Bravais lattices > Introduction (Attention Grabber) Learning Objectives On completion of this chapter you will be able to know about: Crystalline and non crystalline solids Crystal system A solid is that form of matter that possesses rigidity and hence possesses a definite shape and a definite volume. There are two types of solids: Crystalline solids Solids with a definite geometric pattern. Examples: Iron, copper, silver, sulphur etc. are some elements which form crystalline solids. Potassium chloride, sodium nitrate etc are some of the compounds, which are crystalline. Amorphous solids Solids with particles not arranged in a regular fashion. They have only short range order or even the particles are disordered in some cases. Examples: Glass. Rubber and Plastics Material prepared by: < Physics Faculty> Topic No: < 1 > Page 1 of 10 < Engineering Physics -I > < Crystal Physics – Lattice, Unit Cell and Bravais lattices > Classification of Crystalline Solids Ionic Molecular Covalent and Metallic Classification of Crystalline solids Some substances adopt different structural arrangements under different conditions. Such arrangements are called Polymorphs. Example: Diamond and graphite are two different polymorphic forms of carbon. Material prepared by: < Physics Faculty> Topic No: < 1 > Page 2 of 10 < Engineering Physics -I > < Crystal Physics – Lattice, Unit Cell and Bravais lattices > The study of the geometric form and other physical properties of crystalline solids by using X-rays, neutron beams and electron beams constitute the science of crystallography. Crystallography is mainly used to determine the internal atomic arrangements in crystal, bonding and their strength. Lattice Points: Lattice points denote the position of atoms or molecules in the crystals. Space Lattice: The three dimensional space lattice may be defined as an infinite array of points in three dimensions in which every point has an identical environment as any other point in the array (Fig.1). Fig.1. Thus a crystal lattice refers to the geometry of set of points in space. As infinite three dimensional arrays of points showing how atoms or molecules are arranged in a crystal is known as space lattice or lattice array. In the lattice array, every point has surroundings or environment identical to that every point in the array. Basis and Crystal Structure: In order to convert the geometrical array of points (i.e. the lattice) in a crystal Material prepared by: < Physics Faculty> Topic No: < 1 > Page 3 of 10 < Engineering Physics -I > < Crystal Physics – Lattice, Unit Cell and Bravais lattices > structure, we must locate atoms or molecules on the lattice points. The repeating unit assembly- atom, molecule, ion or radical – that is located at each lattice point is called basis. Thus the basis is an assembly of atoms identical in composition, arrangement and orientation. Thus a crystal structure is formed by the following logical relation. Space lattice + basis = crystal structure Unit Cell: The crystal structure of a material or the arrangement of atoms in a crystal structure can be described in terms of its unit cell. The unit cell is a tiny box containing one or more motifs, a spatial arrangement of atoms. The units cells stacked in three-dimensional space describes the bulk arrangement of atoms of the crystal (Fig.2.). Fig.2. Unit cell definition using parallelepiped with lengths a, b, c and angles between the sides given by α, β,γ. The size and shape of a unit cell is determined by the lengths of the edges of the unit cell (a, b and c) and by the angles α, β and γ between the edges b and c, c and a, and a and b respectively. The intercepts a, b and c define the dimensions of a unit cell and are known as its primitives or characteristic intercepts on the axes. These three quantities a,b and c are also called the fundamental translational vectors. Material prepared by: < Physics Faculty> Topic No: < 1 > Page 4 of 10 < Engineering Physics -I > < Crystal Physics – Lattice, Unit Cell and Bravais lattices > The primitives a, b and c and the interfacial angles α, β and γ are the basis lattice parameters because they determine the form and actual size of the unit cell and hence the space lattice. The unit cell formed by the primitives a,b and c is called primitive cell. In a primitive cell, there is only one lattice point. If there are two or more lattice points then it is not a primitive cell. In case of simple cubic crystal lattice, the primitive cell and unit cell are equal since it has only one lattice point in its unit cell. But most of the unit cells of various crystal lattice contain two or more lattice points and it is not necessary that the unit cell should be equal to the primitive cell. Crystal Systems The symmetry of the axial distances (a, b, c) and also the axial angles between the edges (α, β and γ) the various crystals (Table-1) can be divided into seven systems (Fig.3) These are also called crystal habits. Table -1 Material prepared by: < Physics Faculty> Topic No: < 1 > Page 5 of 10 < Engineering Physics -I > < Crystal Physics – Lattice, Unit Cell and Bravais lattices > Fig.3. Material prepared by: < Physics Faculty> Topic No: < 1 > Page 6 of 10 < Engineering Physics -I > < Crystal Physics – Lattice, Unit Cell and Bravais lattices > Bravais lattices When the crystal systems are combined with the various possible lattice centerings, we arrive at the Bravais lattices. They describe the geometric arrangement of the lattice points, and thereby the translational symmetry of the crystal. In three dimensions, there are 14 unique Bravais lattices which are distinct from one another in the translational symmetry they contain. All crystalline materials recognized until now (not including quasicrystals) fit in one of these arrangements. The Bravais lattices are sometimes referred to as space lattices. The crystal structure consists of the same group of atoms, the basis, positioned around each and every lattice point. This group of atoms therefore repeats indefinitely in three dimensions according to the arrangement of one of the 14 Bravais lattices. The characteristic rotation and mirror symmetries of the group of atoms, or unit cell, is described by its crystallographic point group. The following fourteen different types of lattices are known as Bravais lattices (Fig.4). Fig.4 Material prepared by: < Physics Faculty> Topic No: < 1 > Page 7 of 10 < Engineering Physics -I > < Crystal Physics – Lattice, Unit Cell and Bravais lattices > Fig.4 Material prepared by: < Physics Faculty> Topic No: < 1 > Page 8 of 10 < Engineering Physics -I > < Crystal Physics – Lattice, Unit Cell and Bravais lattices > Check your understanding 1. The three axes of a crystal lattice are mutually perpendicular and two of the lattice parameters are equal. The crystal systems is (a) Cubic (b) Tetragonal (c) Orthorhombic (d) hexagonal 2. Which of the following is an example of tetragonal lattice? (a) Calcite (b) Potassium (c) Mercury Chloride (d) None Check the correct answers on page: 9 Summary On completion of this chapter you have learned that: 1. Crystals have directional properties and are anisotropic substances 2. The regular arrangement of the space positions of the atoms in a crystal is called space lattice. 3. A crystal structure is developed by the combination of space lattice and basis. 4. A unit cell is defined as the volume of a solid from which the entire crystal can be constructed by translational repetition in three dimensions. 5. The intercepts on the crystallographic axes a, b and c which define the dimension of a unit cell and the interfacial angles α, β and γ are the basic lattice parameters. 6. There are seven crystal systems which can form fourteen Bravais lattices in the three dimensions. Activity Prepare the models of seven crystal systems Suggested Reading 1. Engineering Physics by Dr.P.K.Palanisamy, Scitech Publishers, Chennai-17 Material prepared by: < Physics Faculty> Topic No: < 1 > Page 9 of 10 < Engineering Physics -I > < Crystal Physics – Lattice, Unit Cell and Bravais lattices > 2. Engineering Physics by Dr.G.Senthilkumar, VRB Publishers, Chennai-92 Answers to CYU. 1. (b) 2. (d) Material prepared by: < Physics Faculty> Topic No: < 1 > Page 10 of 10