Flame Test Lab
advertisement

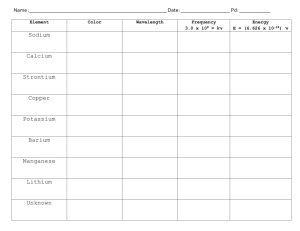
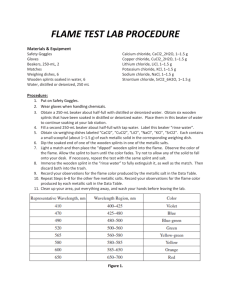
Name: ______________________________________ Date: _______ Period: ____ Flame Test Introduction: When atoms or ions in the ground state are heated to high temperatures, some electrons may absorb enough energy to allow them to “jump” to higher energy levels. These excited state electrons are unstable and they will “fall” back to their normal positions of lower energy. As the electrons return to the ground state, the energy that was absorbed is emitted in the form of electromagnetic energy. Some of this energy may be in the form of visible light. When electrons become excited, the flame will only produce color for a few seconds at most. Many elements produce flames of a characteristic color when they come in contact with the flame of the burner. Using this method, scientists can determine some elements in an unknown mixture or solution. A flame test is made by taking a wooden splint soaked in a chemical of the known element, heating it in a flame, and noting the distinctive color of the flame produced by the vaporized salt. Important vocabulary in this lab includes: • Excited State - when an electron gains enough energy to temporarily leave its ground state orbital and move to an orbital of higher energy. • Ground State - the level of energy that an electron will be in when it is not excited. Objective: To observe the characteristic colors produced by metallic ions when heated in a flame, and to identify an unknown ionic compound by means of its flame test. Materials and Equipment: o o o o 8 Wooden splints soaked in Ionic Solution (NaCl, KCl, LiCl, CaCl2, CrCl2, CuCl2, BaCl2, NiCl2) 1 Wooden splint with unknown Bunsen burner Waste Beaker Procedure: 1. Obtain 8 wooden splints that have been soaking in the metal salt solutions. Be sure to label each wooden splint with the names of the salts so they are not mixed up. 2. Light the Bunsen burner. Be sure to avoid a yellow flame. Name: ______________________________________ Date: _______ Period: ____ 3. Carefully place the end of the wooden splint that was soaked in the metal salt solution at the top of the inner blue flame. Record the color and intensity (bright/faint) of the flame in the data table. The color given off by the salt is the initial color observed, not the yellow-orange color produced by the burning wood. • Use your iPad to record each test to aid in your analysis and for discussions in class. 4. Repeat with the other 7 salts. Be sure to record the colors as precisely as possible. 5. If more observations are needed, tell me for additional resources. Otherwise, discard the wooden splints at the end of the experiment. Data: Ionic Compound LiCl Metal Ion Color in Flame Wavelength (nm) Wavelength (m) Energy (J) KCl CoCl2 CaCl2 CrCl2 CuCl2 BaCl2 NiCl2 Unknown Calculations: In order to calculate the amount of energy found in emitted photon of light, seen as the color of light you observed you will need to use the following equation. Δ𝐸𝐸 = 𝑐𝑐 ∗ ℎ 𝜆𝜆 c: Speed of Light - 3x108 m/s h: Planck's Constant - 6.626x10-34 J*s λ: wavelength - from observation in mm • Remember you will need to use the conversion 1m = 1x109nm Name: ______________________________________ Date: _______ Period: ____ Questions: 1. What inaccuracies may be involved in using flame tests for identification purposes? The electromagnetic spectrum is shown below. Recall that energy is proportional to frequency, while frequency is inversely proportional to wavelength. Use this information to answer questions 2-5 below. 2. List the colors observed in this lab from the highest energy to the lowest energy. 3. List the colors observed in this lab from the highest frequency to the lowest frequency. 4. List the colors observed in this lab from the shortest wavelength to the longest wavelength. 5. What is the relationship between energy, frequency, and wavelength? Name: ______________________________________ Date: _______ Period: ____ 6. Based on the results of your experiment, what metal was found in the unknown? Explain your reasoning. 7. Do you think we can use the flame test to determine the identity of multiple unknowns within a mixture? Why or why not? 8. How are electrons “excited” in this part of the experiment? What does it mean when the electrons are “excited”? 9. Why do different chemicals emit different colors of light? 10. In this lab, you observed that each element emits a unique color of light when heated in a flame. If these light emissions were examined through a prism, you would observe that the emitted light is actually composed of different wavelengths of light that may lie in the violet region, the green region, or the red region of the visible spectrum. Each element has a unique emission spectrum. Research how scientists apply these emission spectra to investigate the chemical composition of stars? What is the emission spectrum of the sun and what does this spectrum reveal about the types of elements in the sun?