The Light Reactions (Chapter 22)
advertisement

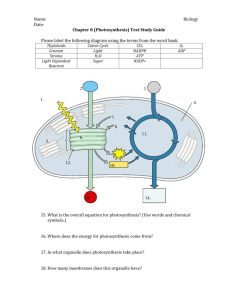
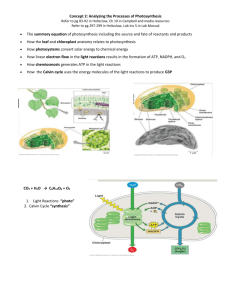
Chapter 22 – The Light Reactions (Problems 1,2,4,5,8-10,13-19) Energy + 6H2O + 6CO2 Light C6H12O6 + O2 Glycolysis/CAC ETC/OX-Phos Photosynthesis C6H12O6 + O2 6H2O + 6CO2 + Energy ATP • Photosynthesis: a process that converts atmospheric CO2 and H2O to carbohydrates • Solar energy is captured in chemical form as ATP and NADPH • ATP and NADPH are used to convert CO2 to hexose phosphates • Phototrophs: photosynthetic organisms (some bacteria, algae, higher plants) Respiratory ETC Energy + 6H2O + 6CO2 Light C6H12O6 + O2 Glycolysis/CAC ETC/OX-Phos Uphill Energy Process Downhill Energy Process Photosynthesis ETC Photosynthesis C6H12O6 + O2 6H2O + 6CO2 + Energy ATP Light and dark reactions • Both processes can occur simultaneously Reactions that require light (light reactions): H2O + ADP + Pi + NADP+ O2 + ATP + NADPH + H+ Reactions which do not require light (dark reactions): CO2 + ATP + NADPH + H+ Sum: CO2 + H2O (CH2O) + ADP + Pi + NADP+ (CH2O) + O2 22.1 Photosynthesis Takes Place in Chloroplasts Three membranes, and three “spaces”. H2O + ADP + Pi + NADP+ → O2 + ATP + NADPH + H+ CO2 + ATP + NADPH + H+ → (CH2O) + ADP + Pi + NADP+ 22.2 Photosynthesis Transforms Light Energy into Chemical Energy I II I II I II I II Structures of Chlorophyll and bacteriochlorophyll • Chlorophylls - usually most abundant and most important pigments in light harvesting • Contain tetrapyrrole ring (chlorin) similar to heme, but contains Mg2+ • Chlorophylls a (Chl a) and b (Chl b) in plants • Bacteriochlorophylls a (BChla) and b (BChlb) are major pigments in bacteria Light harvesting complexes enhance the efficiency of photosynthesis Accessory pigments Reaction centers of the photosystems • PSI and PSII each contain a reaction center (site of the photochemical reaction) • Special pair: two chlorophylls in each reaction center that are energized by light • In PSI special pair is: P700 (absorb light maximally at 700nm) • In PSII the special pair is: P680 (absorb light maximally at 680nm) Some Plants Produce Toxins; e.g. Potatoes 22.3 Two Photosystems Generate a Proton Gradient and NADPH Photosystem I: Ferredoxin and NADPH Production NADP+ NADPH H + Problem: Fd accepts and donates electrons one at a time, but NADP+ accepts a pair of electrons. Solution: Fd NADP+ reductase. Fd NADP+ reductase uses the co-factor FAD which can accept electrons one at a time from Fd and donate a pair of electrons to NADP+ to form NADPH Photosystem I P700* NADP+ + H+ P700 Fd P700+ Fd-NADP+ oxidoreductase (via FAD/FADH2—why?) NADPH From PC Reduction of NADP+ (Eo’ = -0.32 V) by Fd (Eo’= -0.43 V) is catalyzed by ferredoxin-NADP+ oxidoreductase. Photosystem II: Transfers Electrons to PSI and Generates Proton Gradient PSII: Reduction, excitation and oxidation of P680 • P680 special-pair pigment of PSII x 2 • P680+ is reduced by e- derived from oxidation of H2O • Light energizes to P680*, increasing its reducing power Pha e2H+ + e- PAQH2 2H2O → O2 + Via OEC 4H+ (bound) + 4e- PBQH2 (soluble) PAQ Photosystem II: Electrons are Transferred from Q to Pc via Cytbf, and then from Pc to PSI Cytbf PSI Transfer of electrons from QH2 to plastocyanin QH2 + 2Pc(Cu2+) Cytbf → Q + 2 Pc(Cu+) + 2H+ Thylakoid lumen Photosystem II: The Evolution of O2; the OEC of PSII 2H2O → O2 + 4H+ + 4eVia OEC 2H2O → 4e- + 4H+ + O2 To P680+ D1-Tyr-OH → D1-Tyr-O. + eD1-Tyr-O. + e- → D1-Tyr-OH From Mn The manganese center of PSII Cooperation between PSI and PSII 22.4 A Proton Gradient Drives ATP Synthesis 2H2O + 2NADP+ + 10H+stroma → O2 + 2NADPH + 12H+lumen 3ADP3- + 3Pi2- + 3H+ + 12H+lumen → 3ATP4- + 3H2O + H+stroma 2NADP+ + 3ADP3- + 3Pi2- + H+ → O2 + 2NADPH + 3ATP4- + H2O Cyclic Electron Flow Stroma Thylakoid lumen When NADPH/NADP+ ratio is high ….. Organization of Photosynthesis Components Stroma Thylakoid lumen Organization optimizes location PSI for NADPH production in stroma, PSII for increasing proton concentration in thylakoid lumen, and ATP synthase for production of ATP in stroma PSII (QH2 Inhibition) Herbicides PSI e acceptor