Ocean County College Department of Biology Basic Chemistry for
advertisement

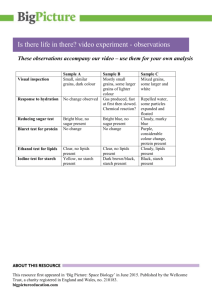
Ocean County College Department of Biology Basic Chemistry for Investigating Living Systems Submitted by Shai Elkayam # 0303810 Date Submitted: 09/17/2014 Date Performed: 09/15/2014 Lab Section: BIOL – 161 – DL1 Course Instructor: Professor M. LaBella Purpose To explain how colorimetry can be used to qualitatively detect cellular chemical components. To chemically differentiate between proteins, sugars, starches, and lipids. To identify the roles of molecular components in living systems. To comprehend the value using a systematic approach to research. To describe why hypothesis, controls, standards, and quality control are important in scientific research. • To suggest scenarios where colorimetry can be applied to medical and industrial applications. • • • • • Materials Distilled water, table salts, vegetable oil, potato, onion, tap water, egg, IKl indicator 2.1%, starch solution 1% stabilized, a-­‐Amylaze powder, Biuret reagent, Benedict’s reagent, Sudan lll, Glucose Solution 20%, pH 7.0 Buffer (Yellow). Discussion and Review All living material, whether it is mitochondria, cytoplasm, nuclear material, DNA, chloroplasts, or other type of cellular or intra-­‐cellular component, consist of one of four major types of molecules: starch and sugars (carbohydrates), proteins, fats (lipids), nucleic acids (including DNA and RNA). Colorimetry is a type of analytical technique used to determine the presence or absence of a molecule such as lipids and sugars through qualitative analysis. It can be used in quantitative determinations. The term colorimetry refers to the detection of a molecule by the color change observed in the prepared sample as the result of the presence of the molecule of interest. The molecule of interest is also referred to as the sample to as the test substance. In colorimetry a reagent is added to the liquid sample and forms a color complex with the molecule for which it is specific. The presence of a molecule of interest is detected by a color change in the sample. There are a number of reagents which are specific to the detection of a given molecule. Procedure of preparation prior to performing Lab Exercise 1% Albumen Solution: made of mixed 2mL egg white with 18mL pH buffer solution. Calculation: egg white contain 10% albumen protein, so 2mL egg white x 10% = 0.2 mL albumen. (0.2 mL / 20 mL solution) x 100 = Albumen solution 1%. Alpha-­‐amylase solution: made of alpha-­‐amylase powder mixed with distilled water. 0.4% Potato Starch solution: made of 5mL starch solution diluted with 12.5 mL distilled water. Calculation: (1% x 5 mL potato starch solution) / 12.5 mL water potato starch solution = 0.4% 1% Glucose solution: 1mL of 20% glucose solution diluted with 20m L distilled water. Calculations: (20% glucose/mL water) / 20mL water = 1% glucose/mL Onion juice solution: juice from fresh chopped onion. Potato juice solution: juice from fresh chopped potato. Exercise 1: Testing for the Presence of Proteins in Cells Procedure When protein molecules are present, Biuret Reagent reacts with the protein to form a violet color. 6 test tubes, one control and five different solutions, were filled from 1.0 cm from the bottom. Data Table 1: Biuret Reagent Test for p rotein Test Tube Contains 1 2 3 4 5 6 Water Albumen Amylase Potato Starch Onion Juice Potato Juice Hypothesis: Contains Protein Final Color Yes or No? No Almost colorless Yes Yellow -­‐ Violet Yes Light violet No Almost colorless No No Yellow -­‐ purple Yellow -­‐ purple (Student to fill in) Is test substance present/ Absent? Absent Yes Yes Absent Little concentration Little concentration Labpaq questions A. What is the test substance? Answer: Protein B. Which test tube represents the control? Why? Answer: the distilled water represents the control because we know that it has no substance in it, thus any change in the other tubes can be compared to the control tube. C. Which test tube contained the most test substance? Answer: the albumen has the most protein. D. Other than the control, which test tube contained the least test substance? Answer: potato starch E. Did the results agree with your initial hypothesis in every case? Why or why not? Answer: I assumed that except albumen and amylase, potato juice and onion juice both will not show protein in the test. However, since potato and onion juice have low amounts of protein (1% and 2%), the protein substance show little concentration of protein in their tubes. F. What are your conclusions about your results? Answer: Certain foods, like eggs, are rich in protein such as the albumen in the egg-­‐white, and some enzymes, such as Amylase, are made of protein. Every living system contains some amount of protein since they are made of cells, but the Biuret Reagent is not able to detect very small amount of protein, such as in the potato and the onion. G. If the color change is not as you expected, what might be the reasons? Answer: it indicates that the test substance is not present in that sample. H. Add another 5 drops of Biuret Reagent to each test tube and stir as before. Do your results change? Answer: No change. Labpaq discussion A. What is the purpose of this exercise? Answer: the purpose of this exercise is to use Biuret Reagent as a colorimetric indicator of the presence of protein in a number of samples. When protein molecules are present, Biuret react with the protein to form violet color. B. Why is it important to clean droppers and equipment between chemical uses? Answer: Clean equipment is important to prevent cross-­‐contamination which can skew the test results. C. What other types of foods or substances contain high levels of protein? Answer: Muscle fibers, hair, nails, skin, enzymes, and certain foods. D. Suggest a situation where you might use the Biuret Reagent colorimetric test. Answer: conditions such as diabetes or chronic kidney failure can be detected using urine test. These types of diseases allow proteins to path through the kidneys and end up in the urine which can then be found by a protein test as colorimetry with Biuret Reagent. E. What other types of analytical procedures detect the presence of proteins? Answer: Dumas method, Kjeldhal method, Lowry protein assay, Turbidimetry, and Bradford protein assay. Exercise 2: Testing for the presence of starch in cells Procedure When starch molecules are present, Iodine Indicator Solution reacts with the starch to form a black-­‐blue color. 6 test tubes, one control and five different solutions, were filled from 1.0 cm from the bottom. Observations Data Table 2: Iodine Solution Test for S tarch Test Tube Contains 1 2 3 4 5 6 Water Albumen Amylase Potato Starch Onion Juice Potato Juice Hypothesis Contains Starch Final Color Yes or No? No Orange-­‐yellow No Orange-­‐yellow No Orange-­‐yellow Yes Black-­‐blue No Yes Orange-­‐yellow Black-­‐blue (Student to fill in) Is test substance present/ absent? Absent Absent Absent Yes Absent Absent Labpaq questions A. What is the test substance? Answer: Starch. B. Which test tube represents the control? Why? Answer: the distilled water represents the control because we know that it has no substance in it, thus any change in the other tubes can be compared to the control tube. C. Which test tube contained the most test substance? Answer: Potato starch. D. Other than the control, which test tube contained the least test substance? Answer: albumen and amylase. E. Did the results agree with your initial hypothesis in each case, why or why not? Answer: the results agreed with my initial hypothesis. Albumen and amylase are proteins so they do not contain starch. Onion is not a starchy vegetable (1.1% average protein) so I did not suspect that there is any significant amount of starch. And potato juice and potato starch are both rich in starch and the colorimetry test proved that by presenting the expected black color. F. What are your conclusions about the results? Answer: there are different ways in nature to save carbohydrate as energy for growth. Onion save carbohydrates in the form of sugars while potato saves carbohydrates in the form of starch (large number of glucose units joined by glycosidic bonds). G. If the color change is not as you expected it to be, what might be the reasons? Answer: all the colors came as expected. Labpaq discussion A. What is the purpose of this exercise? Answer: since iodine solution reacts with carbohydrates to form a blue-­‐black color, a substance that contains carbohydrates can be detected by this colorimetry test. B. What other types of foods or substances contain high levels of starch? C. Answer: starch produced by most green plants as an energy store and is contained in large amounts in staple foods as potatoes, wheat, maize (corn), and rice. D. Suggest a situation where you might use the iodine colorimetric test. Answer: test for starch concentration in the gastrointestinal system can be made with the starch colorimetry test. E. What other types of analytical procedures detect the presence of starch? Answer: methods like chromatographic method, gravimetric method and electrographic method. Exercise 3: Testing for the presence of sugar in cells Procedure: When sugar molecules are present, Benedict’s reagent (copper sulfate) reacts with the sugar to form a green color (lowest concentration) → orange → red → brown color (highest concentration). 6 test tubes, one control and five different solutions, were filled from 0.5 cm from the bottom. Observations Data Table 3: Benedict’s Reagent Test for S ugar (Student to fill in) Hypothesis Is test substance present/ Contains Sugar Test Tube Contains Final Color absent? Yes or No? No Translucent blue Absent 1 Water Yes Red-­‐brown Yes – high concentration Glucose No Blue Absent 3 Albumen No Blue Absent Potato 4 Starch No Orange-­‐red Yes – small concentration 5 Onion No Green-­‐orange Yes -­‐trace amount 6 Potato Juice Labpaq questions A. What is the test substance? Answer: Sugar B. Which test tube represents the control? Why? Answer: the distilled water represents the control because we know that it has no substance in it, thus any change in the other tubes can be compared to the control tube. Since the tube is translucent blue and it has no sugar and orange-­‐red color indicates sugar, the right comparison can be made to determine if sugar is present. C. Which test tube contained the most test substance? Answer: the tube with the glucose. D. Besides the control, which test tube contained the least test substance? Answer: the tube with the potato starch had the least amount of sugar. E. Did the results agree with your initial hypothesis in every case? Why or why not? Answer: my hypothesis for onion and potato juice is that not enough sugar will be indicated by the test. However, the Orange-­‐red color after the reagent was added indicated that both the onion and the potato juice tubes showed small amount of sugar in the test. F. What are you conclusions about the results? Answer: Benedict's reagent is used in a test for the presence of reducing sugars, so the positive test in the glucose tube indicates that glucose is a reducing sugar which reacts with Benedict’s reagent. G. If the color change is not as expected, what might be the reasons? Answer: it can be the result of cross-­‐contamination and/or wrong amounts of solutions and reagent. Labpaq discussion A. What is the purpose of this exercise? Answer: to use colorimetry with the Benedict’s reagent to qualitatively detect sugar molecules in various solutions. Benedict’s reagent test is positive for sugar when the tested solution color is changed to orange or red or brown. B. Suggest a scenario where you might use the Benedict’s reagent colorimetric test. Answer: one example is to use this reagent to test for blood sugar level for people with diabetes. C. How might one determine whether the potato or onion contains more sugar? Answer: The potato solution color turned to green-­‐orange color and the onion solution turned to orange-­‐red color turned when the Benedict’s reagent was introduced to these tubes. Thus, by the reagent know color scale of green color (lowest concentration) → orange → red → brown color (highest concentration) – we could determine that onion cells have more sugar than potato cells. D. What other types of foods or substances contain high levels of sugar? Answer: milk, fruits, sugar cane, and honey for example. E. What other types of analytical procedures detect the presence of sugars? Answer: electrophoretic method, gravimetric method, and chromatographic method for example. Exercise 4: Testing for the presence of lipids in cells Procedure When lipids molecules are present, Sudan lll stain dissolve when in contact with lipids. 6 drops of the Sudan lll stain were placed on a filter paper, and after they dried up, a circle was drawn with a pencil around each stain drop. Then, one drop from 6 different tested solutions and liquids were placed on the dried stain spots. If the stain spot dissolves and pushed beyond the circle, it indicates that the tested substance has lipids because like – dissolves -­‐ like, and the Sudan lll stain, as a fat soluble dye, was dissolved. Observations Data Table 4: Lipid Test Results Hypothesis: Contains lipids, or Macromolecule Being Tested not? Potato Starch No Onion Juice No Vegetable Oil Yes Distilled Water No Albumen No Potato Juice No Results from Test No No Yes No No No Labpaq questions A. What is the test substance? Answer: lipids B. Which test tube represents the control? Answer: the distilled water represented the control since we know that lipids are not water soluble, and it will not dissolve in the Sudan lll stain. C. Which test tube contained the most test substance? Answer: vegetable oil. D. Other than the control, which test tube contained the least test substance? Answer: the onion juice, potato starch, and amylase. E. Did the results agree with your initial hypothesis in every case? Explain why or not. Answer: yes, the results agreed with my initial hypothesis in every case. Labpaq discussion A. What is the purpose of this exercise? Answer: the purpose of the exercise was to qualitatively detect lipids in some cellular chemical compounds. Sudan lll is a fat-­‐soluble dye used for staining lipids that helps to detect lipids when they placed in this stain since Sudan lll dissolves when reacts with lipids. In this experiment, the lipid is dissolved in Sudan lll and spread outside of the circle. B. Explain the molecular basis as to why Sudan III can be used to detect the presence of lipids, but not sugar or proteins. Why can Biuret, Benedict's and Iodine Reagents detect the presence of proteins, starches, and sugars, but not lipids? Answer: It has lots of rings, which are hydrophobic (the avoid water). The fatty acid tails of lipids are also hydrophobic and just like hydrophilic molecules will dissolve in water, hydrophobic molecules will dissolve in other hydrophobic materials -­‐ in this case, lipids C. What other types of foods or substances contain high levels of lipids? Answer: cooking oil, nuts, and fat tissues. D. What other types of analytical procedures detect the presence of lipids? Answer: Babcock method, Gerber method, and detergent method. E. Fill in the summary Table 5 in the Lab Report Assistant section, by noting which complexing reagents (dyes) can be used to determine which cellular components. Use + to indicate that the dye will detect the component, and – to indicate that it will not detect the component. Data Table 5: Testing the Chemical Composition of Cells Indicate a + mark if the reagent reacts with test substance Indicate a -­‐ mark if the reagent does NOT react with test substance Test Substance Biuret Solution Iodine Stain Benedict’s Reagent Protein + -­‐ -­‐ (Albumen) Sugar -­‐ -­‐ + Starch -­‐ + -­‐ Lipid -­‐ -­‐ -­‐ Sudan III -­‐ -­‐ -­‐ + References: 1. Hands-­‐On Labs, Inc., Experiment: Basic Chemistry for Investigating Living Systems 2. Lisa A. Urry, Michael L. Cain, Steven A. Wasserman (2013), Biology in Focus, Campbell