AP Biology Quantitative Skills - The Bronx High School of Science
advertisement
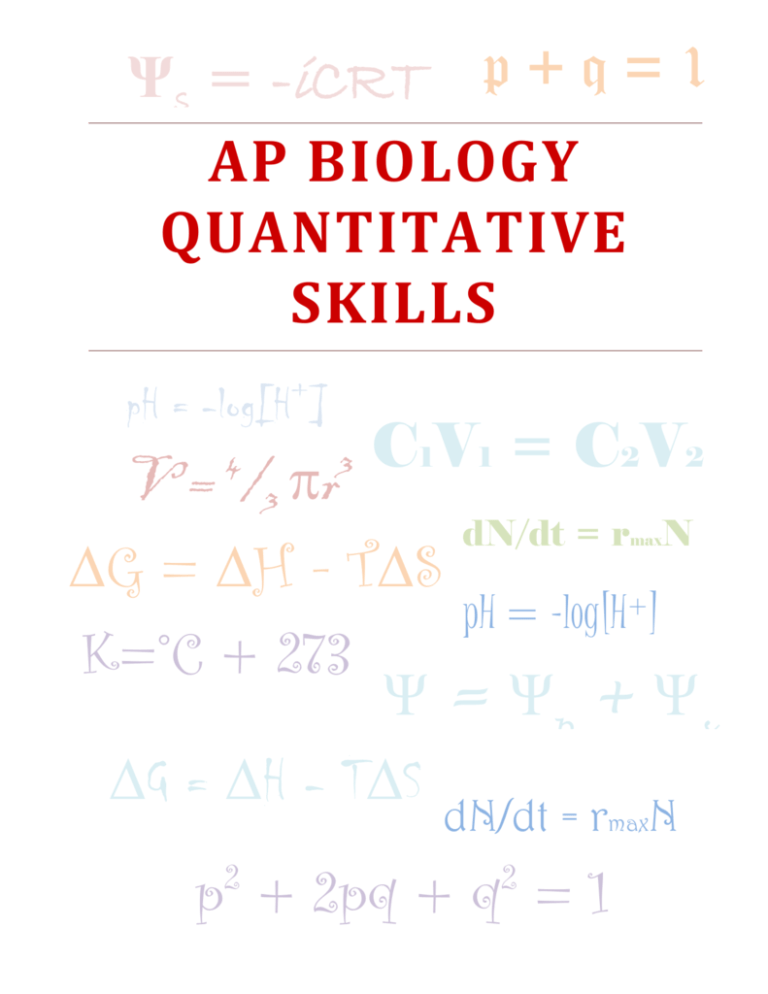
Ψs = -iCRT p + q = 1 AP BIOLOGY QUANTITATIVE SKILLS + pH = -log[H ] C1V1 = C2V2 V = /3 r dN/dt = r N G = H - TS pH = -log[H+] K=°C + 273 Ψ = Ψp + Ψs G = H - TS 4 3 max dN/dt = rmaxN p + 2pq + q = 1 2 2 CONTENTS AP Biology Equations and Formulas ......................................................................................................................................3 Graphing .............................................................................................................................................................................................5 Data Analysis ....................................................................................................................................................................................7 Hypothesis Testing ........................................................................................................................................................................7 Mathematical Modeling................................................................................................................................................................8 Worksheet #1: Basic Statistical Tests ....................................................................................................................................9 Worksheet #2: Chi-Square and Punnett Square ............................................................................................................. 10 Worksheet #3: Hardy-Weinberg A....................................................................................................................................... 11 Worksheet #4: Hardy-Weinberg B ....................................................................................................................................... 12 Worksheet #5: Populations A................................................................................................................................................. 13 Worksheet #6: Populations B................................................................................................................................................. 14 Worksheet #7: Temperature Coefficient ........................................................................................................................... 15 Worksheet #8: Dilutions .......................................................................................................................................................... 16 Worksheet #9: SA:V.................................................................................................................................................................... 17 Worksheet #10: Water Potential .......................................................................................................................................... 18 Worksheet #11: Gibbs Free Energy Basics ....................................................................................................................... 19 Worksheet #12: Gibbs Free Energy Application ............................................................................................................ 21 Worksheet #13: Primary Productivity ............................................................................................................................... 22 Worksheet #14: pH and Metric System ............................................................................................................................. 23 Worksheet #15: Grid-In Practice .......................................................................................................................................... 24 Worksheet #16: Mixed Review .............................................................................................................................................. 26 2|Page AP B IOLOGY E QUATIONS AND F ORMULAS 3|Page 4|Page G RAPHING One of the best ways to communicate the results of a scientific investigation is graphing, or creating an effective visual representation (a graph) of the data that have been counted, measured, and calculated. Investigators often can easily see patterns in a carefully crafted visual display that may not be as readily apparent in a data table of numbers. Visual displays also can clarify how two measured variables affect each other. The AP Biology laboratory manual is designed to encourage students to ask their own questions by designing and carrying out investigations. This process of inquiry requires data analysis and communication of results. The data collected to answer questions generated by students will generally fall into three categories: (1) normal or parametric data, (2) nonparametric data, and (3) frequency or count data. Normal or parametric data are measurement data that fit a normal curve or distribution. Generally, these data are in decimal form. Examples include plant height, body temperature, and response rate. Nonparametric data do not fit a normal distribution, may include large outliers, or may be count data that can be ordered. A scale such as big, medium, small (qualitative) may be assigned to nonparametric data. Frequency or count data are generated by counting how many of an item fit into a category. For example, the results of a genetic cross fit this type of data as do data that are collected as percentages. There are five types of graph you need to understand: bar graphs, box-and-whisker plots, pie charts, scatter graphs and mosaic charts. A. Bar graphs are graphs used to visually compare two samples of categorical or count data. Bar graphs are also used to visually compare the calculated means with error bars of normal data (Figure 1). B. Scatter plots are graphs used to explore associations between two variables visually. C. Box-and-whisker plots allow graphical comparison of two samples of nonparametric data (data that do not fit a normal distribution). D. Histograms, or frequency diagrams, are used to display the distribution of data, providing a representation of the central tendencies and the spread of the data. E. Mosaic charts are modified bar graphs that can show multiple axes simultaneously allowing a more complete comparison. Elements of effective graphing A. Title: must inform the reader about the experiment and tell the reader exactly what is being measured B. Type: the reader must be able to easily discern the type of graph C. Axes: must be clearly labeled with units a. The x axis is the independent variable b. The y axis is the dependent variable c. Intervals must be uniform d. It is not necessary to label every interval e. Labels, including units, should allow the readier ti easily see the information D. Multiple conditions: more than one condition can be present in a graph, however, they need to be clearly labeled and a key must be present. This is the preferable method for comparing multiple experiments that differ by only a single variable. E. Origin: the origin of the graph must be clearly labeled whether it is at (0,0) or not. 5|Page F. Standard error: you may include standard error bars to inform the reader of the accuracy of your data collection. Parametric Test (Normal Data) Non Parametric Tests Frequency Tests (Counts) Mean, Standard Deviation, Error 95% Ci Median, Quartiles, Interquartile Range Percent by Category Bar Graph Box-andWhisker Plot Bar Graph or Pie Chart 2 Groups Unpaired TTest Mann Whitney U-Test ≥ 2 Groups Anova Kruskal-Wallis Test Descriptive Statistics Graph Type Comparative Statistics Independent Samples 2 Groups Paried T-Test Wilcoxon Test ≥ 2 Groups Matched Anova Friedman Test Graph Type Scatter Plot Scatter Plot Mosaic Chart Test For Association Pearson Correlation Spearman Rank Correlation Chi-Square Test For Association Linear Relationship Linear Regression - - Matched Samples Association Statistics Source: Redrawn from “Statistics for AS Biology,” available as part of a download at: http://www.heckmondwikegrammar.net/index.php?highlight=introduction&p=10310 6|Page Chi-Square Test D ATA A NALYSIS 1. Most numeric data falls into one of two categories: measurements or counts 2. Measurements: used to compare a quantitative data point to a universal standard a. Continuous data: infinite number of potential measurements over a given range b. Discrete data: limited number of possible measurements over a given range 3. Count data: recordings of categorical data 4. Descriptive statistics: enables reader to estimate important parameters of the data set and determine a confidence interval a. Examples: standard deviation, mean, median, mode 5. Inferential statistics: uses tools that rely on probability theory and an understanding of distributions to determine precise estimations H YPOTHESIS T ESTING 1. Statistical hypothesis testing focuses on trying to reject a null hypothesis. a. A null hypothesis is a statement explaining that the underlying factor or variable is independent of the observed phenomenon. b. Alternative to the null hypothesis is a statement that the underlying factor or variable is not independent of the observed phenomenon. Possible Outcomes of Hypothesis Testing Investigator action Null hypothesis is true Null hypothesis is false Rejects the null hypothesis Type I error (false positive) Correct Fails to reject null hypothesis Correct Type II error (false negative) 1. We use a critical (p) value (usually 95% sure of answer). a. Using this system, there is only a 5% chance of making a type I error. 2. Chi-square testing ∑ i null hypothesis: there is no difference between (the item being studied) and (random chance) b. degrees of freedom = number of samples - 1 c. use the 0.05 value for your critical value (a) if the value is higher than the critical value- reject null hypothesis (b) if the value is lower than the critical value-fail to reject null hypothesis 7|Page M ATHEMATICAL M ODELING Process of creating mathematical or computer based representations of the structure and interactions of complex systems 1. Models are formed after experimentation and ask these types of questions a. What can we measure b. What should we measure c. What are the relevant variables d. What are the simplest informative models we can build 2. Aspects of models a. Introducing power of the model (a) Approximating real world conditions b. Introducing limitations of the model (a) Assumptions (b) Simplifications 3. Components of mathematical models a. Examine a system to identify which variables seem to be most likely to have an effect b. Develop graphical or physical models to capture the essence of the phenomenon. c. Translate the model into a “word equation.” d. Translate word equations into formal equations e. Implement the model on a computer f. Evaluate, revise, extend the model (a) Look at the basic assumptions, can they be eliminated? (b) Look at the simplification, can it be minimized? (c) Look at the approximation, can it be improved? 8|Page W ORKSHEET #1: B ASIC S TATISTICAL T ESTS Mode = value that occurs most frequently Median = middle value Mean = average Range = dispersion of data points (value obtained by subtracting the smallest observation from the greatest observation) ( xi x ) 2 SD= n 1 s SE x n standard deviation standard mean error ̅ = sample mean n = size of sample s= sample standard deviation o= observed results e=expected results 1 n x xi n i 1 mean Example problem: One of the lab groups collected the following data for the heights (in cm) of their Wisconsin Fast Plants: 5.4 7.2 4.9 9.3 7.2 8.1 8.5 5.4 7.8 10.2 Find the mode, median, mean, and range. Show your work where necessary. Mode(s):___________ ___ Median: ___________ ___ Mean: ___________ ___ Range: ___________ ___ Find the standard deviation by filling in the following table. Heights (x) 5.4 7.2 4.9 9.3 7.2 8.1 8.5 5.4 7.8 10.2 Mean (x ) xx (x x)2 ( x x ) 2 Standard deviation: Interpret the standard deviation in the context of the problem. What is the standard mean error? Calculate it for this data set. 9|Page W ORKSHEET #2: C HI -S QUARE AND P UNNETT S QUARE Formulas: ( o e) 2 e 2 Chi Square o = observed individuals with observed genotype e = expected individuals with observed genotype Degrees of freedom equals the number of distinct possible outcomes minus one Degrees of Freedom p 1 2 3 4 5 6 7 8 0.05 3.84 5.99 7.82 9.49 11.07 12.59 14.07 15.51 0.01 6.64 9.32 11.34 13.28 15.09 16.81 18.48 20.09 Example problem: Wisconsin Fast Plants have two very distinctive visible traits (stems and leaves). Each plant will either have a purple (P) or green (p) stem and also have either have green (G) or yellow (g) leaves. Suppose that we cross a dihybrid heterozygous plant with another plant that is homozygous purple stem and heterozygous for the leaf trait. Make a Punnett square to figure out the expected ratios for the phenotypes of the offspring. Suppose a class observed that there were 234 plants that were purple stem/green leaves and 42 that were purple stem/yellow leaves. Does this provide good evidence against the predicted phenotype ratio? Using your understanding of genetics, what might be one reason why the class got these results? 10 | P a g e W ORKSHEET #3: H ARDY -W EINBERG A Formulas: p2 + 2pq + q2 = 1 p+q=1 p = frequency of the dominant allele in a population q = frequency of the recessive allele in a population For people, being right handed (R) is the dominant trait over being left handed (r). Suppose there is a sample of 20 people that reveals the following genotypes: (RR) (Rr) (Rr) (rr) (RR) (Rr) (Rr) (Rr) (rr) (RR) (Rr) (RR) (RR) (Rr) (RR) (RR) (Rr) (rr) (RR) (Rr) What percentage of the people are right handed? Left handed? Find p and q and interpret each in the context of the problem. Now suppose that we took another sample of 10 people. This time we only know their phenotypes. (Right) (Left) (Right) (Right) (Right) ( Right) (Right) (Right) (Left) (Right) What percentage of the people are right handed? Left handed? Can you find p and q exactly? Why? Estimate p and q and interpret each in the context of the problem. Estimate how many of the right handed people are homozygous and how many are heterozygous. 11 | P a g e W ORKSHEET #4: H ARDY -W EINBERG B Formulas: p2 + 2pq + q2 = 1 p+q=1 p = frequency of the dominant allele in a population q = frequency of the recessive allele in a population Example problem: In 1988 the Garces Memorial High School student body was made up of 90% right handed students. Being right handed (R) is the dominant trait over being left handed (r). What is p and q for the population of 1990 GMHS High School students. Interpret each. Find the percent of the student body in 1990 that are homozygous right handed, heterozygous right handed, and left handed. Fast forward to today at Garces. We took a random sample of 100 students today and found that 18 of them were left handed. What are the new p and q values? How do they compare with the values from 1990? There are many reasons why this apparent change could have occurred. Come up the five you will be expected to know and give an example for each: (Hint: Why did I choose 1988, the year I graduated?) 12 | P a g e W ORKSHEET #5: P OPULATIONS A Rate Population Growth Exponential Growth Logistic Growth dY/dt dN/dt = B – D dN rmax N dt dN K N rmax N dt K dY = amount of change K = carrying capacity Notes B = birth rate D = death rate N = population size rmax = maximum per capita growth rate of population dN N change in population size = = = population growth rate t dt change in time Example 1: There are 300 falcons living in a certain forest at the beginning of 2013. Suppose that every year there are 50 falcons born and 30 falcons that die. What is the population growth rate (include units)? Interpret the value. What is the per capita growth rate of the falcons over a year? Interpret the value. dN rmax N dt c. Fill in the table and construct a graph. Year Population Year 2013 2019 2014 2020 2015 2021 2016 2022 2017 2023 2018 2024 Population Find the average rate of change for the falcon population from 2013 to 2018 (include units). Interpret the value. 13 | P a g e W ORKSHEET #6: P OPULATIONS B Bakersfield had a population of 347,500 in the year 2010. The infrastructure of the city allows for a carrying capacity of 450,000 people. rmax = .9 for Bakersfield. a. Is the current population above or below the carrying capacity? Will the population increase or decrease in the next year? b. What will be the population growth rate for 2010 (include units)? c. What will be the population size at the start of 2014. d. Fill in the following table: Year Population size at start of year Population growth rate (new people added) 2010 2011 2012 2013 2014 e. What happened to the population size over the years? f. What happened to the population growth rate over the years? g. f. Explain your answer from part (e) using what you know about carrying capacity. h. g. Explain your answer from part (e) using the formula: 14 | P a g e dN K N rmax N dt K W ORKSHEET #7: T EMPERATURE C OEFFICIENT k Q10 2 k1 T2=higher temperature T1 = lower temperature k2= reaction rate at T2 k1=reaction rate at T1 Q10=factor by which the reaction rate increases when the temperature increases by 10°C 10 T2 T1 R2 =R1 x Q10 The rate of metabolism of a certain animal at 10ºC, is 27 μL O2 g-1h-1. 1. What are its rates of metabolism at 20, 30, and 40 ºC if the Q10 is 2? If it is 2.5? Temperature ºC 20 30 40 Temperature ºC 20 30 40 Rate2 if Q10 = 2 Rate2 if Q10 = 2.5 2. Graph the two tables above showing the effect of Temp on reaction rate Temperature (ºC) Rate of Metabolism (μLO2 g-1h-1.) 15 10 20 13.42 30 21.22 Q10 The table above reports the rates of metabolism of a species at a series of ambient temperatures: 3. Calculate the Q10 values for each temperature interval 4. Within which temperature interval (15-20 or 20-30) is the rate of metabolism most sensitive to temperature change? 5. For this species, would a Q10 calculated for 15 to 30 ºC be as useful as several for smaller temperature ranges? Calculate that Q10 as part of your answer. The reaction rate for a certain process at 14 ºC is 15 units/time. What would be the reaction rate at 20 ºC if the Q10 = 1? 15 | P a g e W ORKSHEET #8: D ILUTIONS C1V1 = C2V2 aka. M1V1=M2V2 1M AgNO3= 1 mol AgNO3/L C1 = original concentration of the solution, before it gets watered down or diluted. C2 = final concentration of the solution, after dilution. V1 = volume about to be diluted V2 = final volume after dilution For all dilution problems C1> C2, and V1< V2. It makes sense because to dilute, we add water. Joe has a 2 g/L solution. He dilutes it and creates 3 L of a 1 g/L solution. How much of the original solution did he use? What is the molarity of a solution with 360 g glucose in 500 mL of distilled water? Since Joe did such a good time before, the teacher asked Joe to make a set of solutions. For the lab the students need 2-L of each NaCl stock solution at 1.0M, 0.75M, 0.50M, and 0.25M. If the molar mass of NaCl is 58.45 g/mol, what are the directions for each of the solutions. Be specific and show your calculations. 16 | P a g e W ORKSHEET #9: SA:V Surface area to Volume and Water Potential Review Cells throughout the world have variable shapes and sizes. Because of this, and because structure is designed around function, certain shapes are optimal for certain processes. Analyze the following cells (units not to scale), and determine the following… Cell 1 (spherical) where the diameter is 6 mm Vsphere = 4/3 π r3 Vrectangle = l w h Asphere = 4 π r2 Arectangle = Σ (SA for each side) Cell 2 (flat and rectangular) where the height is 0.5mm, length is 4mm, width is 2mm Cell 3 (cube) where side length is 6 mm Cell Surface area Volume Surface area to Volume Ratio Cell 1 Cell 2 Cell 3 A) What is the surface area to volume ratio of each cell? Complete the table above. B) Conclusion: Compare the ratios and explain why one cell would be more efficient than another. C) If the volume of two cells are identical, but one is a sphere and the other a cube, what are their respective surface areas? Use an arithmetical example. D) Are you made of lots of large cells or lots of small cells? Why? How do you grow in height? E) Provide 5 specific examples of ways organisms use SA:V ratio to survive. 17 | P a g e W ORKSHEET #10: W ATER P OTENTIAL Ψ= ΨP + ΨS ΨP= pressure potential; ΨS = solute potential ΨS = -iCRT i = ionization constant; C = molar concentration; R = 0.0831; T=Temp (K) The water potential will be equal to the solute potential of a solution in an open container i is 1.0 for sucrose because sucrose does not ionize in water Water potential in potato cells was determined in the following manner. The initial masses of six groups of potato cores were measured. The potato cores were placed in sucrose solutions of various molarities. The masses of the cores were measured again after 24 hours. Percent changes in mass were calculated. The results are shown below Molarity of Sucrose in Beaker 0.0 M 0.2 0.4 0.6 0.8 1.0 Percent Change in Mass 18.0 5.0 -8.0 -16.0 -23.5 -24.0 Graph these data. From your graph, label where the cells were hypotonic and hypertonic. Determine the apparent molar concentration (osmolarity) of the potato core cells. Looking at the water potential equation, Pressure potential is always (positive/negative), while solute potential is always (positive/negative). When Solution potential goes down (gets more negative), water potential (increases/decreases) When Pressure potential goes down (gets smaller), water potential (increases/decreases) When would the pressure in a cell rise? (Under what conditions?) What would happen to the solute potential when Concentration is increased (justify with equation)? WHY? What would happen to the solute potential when Temperature is increased (justify with equation)? WHY? 18 | P a g e What would happen to the solute potential when the dissolved substance is glucose vs. salt (justify with equation)? WHY? Why is water potential important for plants? What are they lacking? Predict what would happen to animal cells placed in 0.0M and 1.0M concentration solution W ORKSHEET #11: G IBBS F REE E NERGY B ASICS ΔG = ΔH - T ΔS What is Entropy (ΔS) = a measurement of ________________________________________________ When ΔS is positive this means there is ________________________________________________ When ΔS is negative this means there is ________________________________________________ What is ΔH? = a measurement of ________________________________________________ When ΔH is positive this means the reaction is ________________________________________________ When ΔH is negative this means the reaction is ________________________________________________ What is Gibbs Free energy? = a measurement of ________________________________________________ When ΔG is positive this means the reaction will happen ____________________________________________ When ΔG is negative this means the reaction will happen ___________________________________________ ΔG (Joules) ΔH (Joules) T (Kelvin) ΔS (J/K) 1000 1100 1200 1300 1400 1500 1600 1700 1800 1900 300 300 300 300 300 300 300 300 300 300 5 5 5 5 5 5 5 5 5 5 What happens to ΔG when ΔH goes up ? WHY? What happens to ΔG when ΔH goes down ? WHY? 19 | P a g e ΔG ΔH T ΔS 1700 1700 1700 1700 1700 1700 1700 1700 1700 1700 300 310 320 330 340 350 360 370 380 390 5 5 5 5 5 5 5 5 5 5 ΔH T ΔS 7500 7500 7500 7500 7500 7500 7500 7500 7500 7500 300 300 300 300 300 300 300 300 300 300 5 10 15 20 25 30 35 40 45 50 What happens to ΔG when T goes up ? WHY? What happens to ΔG when T goes down ? WHY? ΔG What happens to ΔG when ΔS goes up ? WHY? What happens to ΔG when ΔS goes down ? WHY? Complete the sentences below. As the reaction requires less and less energy, its spontaneity will (increase, decrease). As randomness increases, the free energy will (increase, decrease) because ______________ __________________________________________________________________________ 20 | P a g e W ORKSHEET #12: G IBBS F REE E NERGY A PPLICATION Energies are usually given as standard free energies of hydrolysis. For example glucose-6-phosphate + water → glucose + Pi has ΔG° = -4.0 kcal/mole (-16.5 kJ/mole) under standard conditions. Therefore, the opposite reaction, the phosphorylation of glucose, is unfavored. However, the phosphorylation of glucose occurs readily in the cell, catalyzed by the enzyme hexokinase: glucose + ATP → glucose-6-phosphate + ADP + Pi The other half of the phosphorylation reaction is the hydrolysis of ATP to yield ADP and inorganic phosphate (Pi): ATP + H2O → ADP + Pi under standard conditions has ΔG° = -7.3 kcal/mole (-31 kJ/mole). The standard free energy change of the reaction can be determined by adding the two free energies of reaction: Glucose + Pi → glucose-6-phosphate + H2O and ΔG° =+-4.0 kcal/mole Note that the reaction as written is unfavored; its free energy change is positive. Another way of stating this is that the reaction is endergonic, that is, the reaction involves a gain of free energy. For the exergonic hydrolysis of ATP (the reaction involves a loss of free energy): ATP + H2O → ADP + Pi ΔG° = -7.3 kcal/mole The two reactions are summed: Glucose + ATP → glucose-6-phosphate + ADP + Pi ΔG° = -3.3 kcal/mole This is a simple example of energetic coupling, where an unfavorable reaction is driven by a favorable one. Coupling doesn't occur all by itself. In this example, if this experiment were set up so that the ATP would have to be hydrolyzed in one tube and the glucose phosphorylated in another, no coupling would be possible. Coupling can occur only when the partial reactions are part of a larger system. In this example, coupling occurs because both partial reactions are carried out by the enzyme hexokinase. In other cases, coupling can involve membrane transport, transfer of electrons by a common intermediate, or other processes. Another way of stating this principle is that coupled reactions must have some component in common. 1. 2. 3. The “orderliness” of your body is not favored by free energy. Explain (in terms of free energy and disorder) why you need to perform digestion? Why does decomposition of a dead animal happen in terms of energy? What would happen if we increase temperature? Why do we freeze food? Explain why plant cells need light to build sugar (in terms of energy). 21 | P a g e W ORKSHEET #13: P RIMARY P RODUCTIVITY mg O2 0.698mL mL O2 x L mg L mL O2 0.536 mg C fixed mg C fixed x L mL O2 L One can determine Primary Productivity by measuring dissolved oxygen in the water (as it is hard to measure it in the air) 1 ml of O2 = 0.536 mg of Carbon assimilated mg O2/L x 0.698 = ml O2/L; ml O2/L x 0.536 = mg carbon fixed/L 6CO2 + 6H2O → C6H12O6 + 6O2 Fill in the table and Graph Net and Gross Productivity vs. % of light % light DO2 (mg O2/L) Initial Dark 100% 65% 25% 10% 2% 8.4 6.2 10.2 9.7 9.0 8.5 7.1 Gross PP = DO2-dark (mg O2/L) — — Net PP = DO2 -initial (mg O2/L) — — Gross carbon fixed in mg C/L Gross PP x 0.698 x 0.536 — — Using your data table, what seems to be the trend as the % of light decreases? WHY? Using your data table, what seems to be the trend as the % of light increases? WHY? Where would you say this organism is using as much energy as they are making? WHY? Using your table and graph, explain why most of the time there are bigger plants on land than in the sea? Explain this in terms of evolution. 22 | P a g e W ORKSHEET #14: P H AND M ETRIC S YSTEM pH = -log[H+] pOH = -log[OH-] pH + pOH = 14 recall [H+] is really [H3O+] Which is more acidic? [H+] 1.0 x 10-8 or 1.0 x 10-12 Which is more basic? [H+] 1.0 x 10-6 or 1.0 x 10-3 The pH of stomach acid is about 1.5. what is the [H+]? Blood has a pH of about 7.40. What is the [H+]? [H+] 1 000 000 1 000 100 10 1 0.1 0.01 0.001 0.000 001 0.000 000 001 0.005 0.05 0.000 026 Scientific notation pH Metric prefix — — — — — Write answers in scientific notation: (NO CALCULATORS) 4.00x105 x 2.00x103 8.00x107 / 2.00x103 4.00x10-5 x 2.00x10-3 8.00x107 / 2.00x10-3 4.00x105 x 2.00x10-3 8.00x10-7 / 2.00x103 4.00x10-5 x 2.00x103 8.00x10-7 / 2.00x10-3 When you divide in scientific notation, you need to _______________________ the exponents. When you multiply in scientific notation you need to _______________________ the exponents. 23 | P a g e W ORKSHEET #15: G RID -I N P RACTICE You will have questions that use a grid-in. in general, these questions should be fairly straight forward. Here are a few examples. - . / / / . . . . There are 252 deer in a population. There is no net immigration or emigration. If 47 deer die and 32 deer are born in one month, what is the population size at the end of the month? Round to the nearest whole number. Solution: 252-47+32=237 All answers below are correct 2 3 7 - . / / / . . . 2 3 7 . - . / / / . . . . Correct ways to write one-half 0 . 5 include: - . / / / . . . 24 | P a g e . . 5 - . 1 / 2 / / / . . . . - . / / / . . . . SAMPLE QUESTIONS SUITABLE FOR GRID-IN RESPONSES. 1. In snapdragons (Antirrhinum), the phenotype for flower color is governed by two alleles – red (R) and white (W). Heterozygous individuals have pink flowers. Two pink individuals are crossed to produce 465 offspring. Calculate how many of these offspring are expected to have the red phenotype. Round your response to the nearest whole number. 2. The molar concentration of a sugar solution in an open beaker has been determined to be 0.3M. Calculate the solute potential at 27 degrees Celsius. Round your answer to the nearest tenths. 3. The net annual primary productivity of a particular wetland ecosystem is found to be 8000 kcal/m2. If respiration by the aquatic producers is 12,000 kcal/m2 per year, what is the gross annual primary productivity for this ecosystem in kcal/m2 per year? Round to the nearest whole number. Respiration Temp rate 4. Data taken to determine the effect of temperature on the rate of (°C) (per min) respiration in a goldfish is given in the table to the right. Calculate Q10 for 16 16 this data. Round to the nearest whole number. 21 22 5. Joe has a 2 g/L solution. He dilutes it and creates 3 L of a 1 g/L solution. How much of the original solution did he dilute (in L)? Round to the nearest tenths. 6. What is the hydrogen ion concentration of a solution of pH 8? Round to the nearest whole number. 7. What is the SA/V for this cell if r = 3m? Round your answer to the nearest hundredth. 8. A census of birds nesting on a Galapagos Island revealed that 24 of them show a rare recessive condition that affected beak formation. The other 63 birds in this population show no beak defect. If this population is in HW equilibrium, what is the frequency of the dominant allele? Give your answer to the nearest hundredth O2 Time 9. There are 2000 mice living in a field. If 1000 mice are born each month produced (min) and 200 mice die each month, what is the per capita growth rate of mice (mL) over a month? Round to the nearest tenths. 1 2.3 2 3.6 10. Hydrogen peroxide is broken down to water and oxygen by the enzyme 3 4.2 catalase. The data were taken over 5 minutes. What is the rate of 4 5.5 enzymatic reaction in mL/min from 2 to 4 minutes? Round to the nearest 5 5.9 hundreds 11. The following data were collected on the behavior of preschoolers. Some were spanked, some were given time-outs and some were given no discipline. Five years later teachers were asked to keep track of the behavior issues of these children. Find the chi-square. no discipline time-outs spanking # misbehaviors 40 34 26 25 | P a g e W ORKSHEET #16: M IXED R EVIEW 1. Students used D. melanogaster for a genetics lab. They chose to look at two traits to see if they were linked. They chose eye color and wing shape. Red is the dominant eye color and normal is the dominant wing shape. After beginning with a P generation of EEWW and eeww, they used the F2 generation. They got the following results. Red eyes, normal wings: 184 Red eyes, stunted wings: 12 Yellow eyes, normal wings: 45 Yellow eyes, stunted wings: 68 Are these genes linked? Support your answer. 2. Albinism is a rare genetically inherited trait that is only expressed in the phenotype of homozygous recessive individuals (aa). The most characteristic symptom is a marked deficiency in the skin and hair pigment melanin. This condition can occur among any human group as well as among other animal species. The average human frequency of albinism in North America is only about 1 in 20,000. What percentage of people carry this gene? 3. If a cell’s ΨP = 3 bars and its ΨS = -4.5 bars, what is the resulting Ψ? a) The cell is placed in a beaker of sugar water with ΨS = -4.0 bars. In which direction will the net flow of water be? b) The original cell 1 is placed in a beaker of sugar water with ΨS = -0.15 MPa (megapascals). We know that 1 MPa = 10 bars. In which direction will the net flow of water be? 4. A change in pH from 3 to 5 means what in terms of [H+]? 5. What is the surface area of a cube that has the same volume as a sphere with a surface area of 100 cm2? 6. The lab kit you got only has 500 mL of 6.0 M HCl, but you need 500 mL of 0.2M HCl. How could you make what you need without wasting any of the stock solution? 7. The following data were collected on the behavior of preschoolers. Some were spanked, some were given time-outs and some were given no discipline. Five years later teachers were asked to keep track of the behavior issues of these children. Did the punishment reflect improved behavior in upper elementary grades? (You did the math earlier (page , now draw the conclusion!) 26 | P a g e no discipline time-outs spanking # misbehaviors 40 34 26 8. Calculate the mean, median, mode and standard deviation for this data set. 12 14 18 12 14 19 21 8 17 6 9. Graph the following scenario. CORRECTLY! Explain what the graph tells you about each plant. 5 9 1 7 Depth (m) 2 5 10 16 25 30 2 6 Bubbles per minute (Plant A) 29 32 45 32 20 10 12 18 16 8 Bubbles per minute (Plant B) 21 27 40 50 34 20 27 | P a g e 10. In a population of 600 squirrels, the per capita birth rate in a particular period is 0.06 and the per capita death rate is 0.12. a. What is the per capita growth rate of the population? Round to the nearest hundredth. b. What is the actual number of squirrels that die during this particular period? c. What is the actual number of squirrels that are born during this period? 11. The doubling time of a population of plants is 12 years. Assuming that the initial population is 300 and that the rate of increase remains constant, how large will the population be in 36 years? 12. There are 780 turkeys living in Merriam Township, which is 92 acres in size. The birth rate is 0.472 turkeys/year per capita. The death rate is 0.331 turkeys/year per capita. a. What is the population density? Round to the nearest tenth. b. What is dN/dt? Round to the nearest whole number. c. Predict N after one year, assuming dN/dt stays constant. Round to the nearest whole number. 13. One dandelion plant can produce many seeds leading to a high growth rate for dandelion populations. If a population of dandelions is currently 40 individuals and rmax = 0.2 dandelions/month per capita, predict how many dandelions would be in this population after 4 months. Round to the nearest whole number. 14. A hypothetical population has a carrying capacity of 1,500 individuals and rmax is 1.0. Fill out the following table. Round all answers to the nearest whole number. Explain the results. Population Show Work Here 1250 1500 1750 2000 15. Determine the Q10 value for the heart rate in Daphnia, the water flea. 28 | P a g e Temperature (Co) Average Heart Rate (beats per minute) 14 127 20 162 26 197 Population Growth 16. What is the primary productivity of this ecosystem at each of the specified light levels? % light DO2 (mg O2/L) Initial 0% 100% 85% 55% 20% 12% 7.4 5.2 9.2 8.7 8.0 7.5 6.1 Gross PP = DO2-dark (mg O2/L) — — Net PP = DO2 -initial (mg O2/L) — — Gross carbon fixed in mg C/L Gross PP x 0.698 x 0.536 — — 17. Calculate the population sizes between the following groups. Each begins with 1,500 individuals and has a maximum growth rate per capita of 0.4. The first population (population A) does not have a carrying capacity while the second (population B) has a carrying capacity of 50,000. Any population that reaches twice the carrying capacity goes locally extinct. Create a model on Excel that will show the populations for the next 100 years. Challenge: make the model able to accommodate changes to the rmax, initial populations, and carrying capacity. Alternately, you can do the work by hand for 30 years. (Hint: use the equations!) 29 | P a g e




