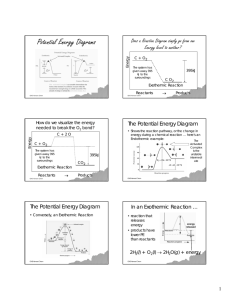
Spontaneity, Entropy, & Gibbs Free Energy What is a Spontaneous
advertisement

Spontaneity, Entropy, & Gibbs Free Energy GHS Honors Chem Spontaneous Processes Spontaneous processes are those that can proceed without any outside intervention. The gas in vessel B will spontaneously effuse into vessel A, but once the gas is in both vessels, it will not spontaneously reverse GHS Honors Chem What is a Spontaneous Process? • Melting Ice? • Freezing of Liquid Water? • Rusting of Iron? GHS Honors Chem Spontaneous Processes Processes that are spontaneous in one direction are nonspontaneous in the reverse direction. GHS Honors Chem Reversible Processes Spontaneous Processes Processes that are spontaneous at one temperature may be nonspontaneous at other temperatures. Above 0°C it is spontaneous for ice to melt. Below 0°C the reverse process is spontaneous. In a reversible process the system changes in such a way that the system and surroundings can be put back in their original states by exactly reversing the process. Changes are infinitesimally small in a reversible process. GHS Honors Chem GHS Honors Chem 1 What Contributes to Spontaneity? Irreversible Processes • Change in Enthalpy (∆H): • Is heat/energy absorbed or given off • Change in Entropy (∆S): • Entropy is a measure of randomness or disorder Irreversible processes cannot be undone by exactly reversing the change to the system. All Spontaneous processes are irreversible. • Temperature of the Reaction (T): GHS Honors Chem GHS Honors Chem Enthalpy’s Contribution to Spontaneity • Many spontaneous processes proceed with a DECREASE in energy, and are Exothermic (produces heat) at 250C and 1 atm. (STP) • 2H2(g) + O2(g) Æ 2H2O(l) ∆H = - 571.6 kJ But it’s not a direct correlation, or a perfect fit … GHS Honors Chem Enthalpy’s Contribution to Spontaneity • Endothermic (takes in heat) reactions that are non-spontaneous at room temp, often become spontaneous at higher temperatures So ∆H’s contribution is tied to the temperature of the reaction … GHS Honors Chem • What is the temperature, or better said, the kinetic energy of the particles in the reaction Enthalpy’s Contribution to Spontaneity Endothermic (takes in heat) reactions that are non-spontaneous at room temp, often become spontaneous at higher temperatures (Increase in energy often increases spontaneity). CaCO3(s) Æ CaO(s) + CO2 ∆H = +17803kJ The decomposition of limestone occurs at 1100 K and 1atm. At 250C & 1atm this reaction does not occur. GHS Honors Chem What about Entropy? What is it? Entropy can be thought of as a measure of the randomness of a system. GHS Honors Chem 2 What about Entropy? What is it? What are the variables that affect Entropy?. • First law of thermodynamics: Energy in the Universe is a constant. Energy cannot be created nor destroyed. • Second law of thermodynamics: Randomness or disorder in the Universe is increasing. • What is Entropy (S)? It is measure of molecular randomness or disorder. Change in entropy is it denoted by the symbol ∆S. GHS Honors Chem GHS Honors Chem Entropy on the Molecular Level What Increases Entropy? • Adding Particles: adding more particles increases the collisions, and the randomness of motion • Adding Energy/Increasing Temperature: velocity of particle motions is increased. • Increasing Volume: particles are allowed to roam in greater space, more random motion How is Entropy Affected by Physical States? Entropy increases with the freedom of motion of molecules: S(g) > S(l) > S(s) http://www.wwnorton.com/chemistry/tutorials/ch13.htm GHS Honors Chem GHS Honors Chem What happens to Entropy in Solutions? Dissolution of a solid: • Ions have more entropy • But, some water molecules have less entropy (they are grouped around ions). Third Law of Thermodynamics • • At absolute zero, a pure substance exists as a perfect crystal, with no molecular movements (no kinetic energy). The entropy of a pure crystalline substance at absolute zero is 0. Usually, there is an overall increase in S. (The exception is very highly charged ions that make a lot of water molecules align around them.) GHS Honors Chem GHS Honors Chem 3 For a chemical reaction, that change in entropy (∆S) under standard conditions is calculated as: Standard Molar Entropies • • Now I know you’re wondering … where do I get these standard Molar Entropy Values?? GHS Honors Chem Whip out your Thermochemical Tables!! These are molar entropy values of substances in their standard states. Standard entropies tend to increase with increasing molar mass. GHS Honors Chem Trends in Standard Entropies • Larger and more complex molecules have greater entropies. Entropy Worksheet • Note for pure elements: GHS Honors Chem GHS Honors Chem How Are Enthalpy (∆H) & Entropy (∆S) Related? Introducing Gibbs Free Energy Gibbs Free Energy & Spontaneity • Gibbs Free Energy is defined as the energy in a system that is available to do useful work. Can the sign of ∆G tell us whether a reaction is spontaneous? Or GHS Honors Chem A + B 1. 2. Æ C If ∆G is negative, the forward reaction is spontaneous. If ∆G is positive, the reaction is spontaneous in the reverse direction. GHS Honors Chem 4 Calculating ∆G 1. Calculating ∆G Standard free energies of formation, ∆Gf° are analogous to standard enthalpies of formation, ∆Hf°. These are located on your Thermochemical Data Chart. ∆G can be calculated from ∆H and ∆S: • This relationship holds true as long as the temperature and pressure remain constant during the reaction. • There are two parts to the free energy equation: • ∆H°— the enthalpy term • T∆S° — the entropy term • The temperature dependence of free energy comes from the entropy term. GHS Honors Chem GHS Honors Chem The Effect of Temperature on ∆G and Spontaneity By knowing the sign (+ or -) of ∆S and ∆H, we can get the sign of ∆G and determine if a reaction is spontaneous. • High T favors the Entropy term GHS Honors Chem • Low T favors the Enthalpy term Calculating ∆G Problem Calculating ∆G • The ∆Hrxn can be calculated from ∆Hf at standard temperature and pressure • The ∆Srxn is also calculated from the ∆S’s at standard temperature and pressure. • The Gibbs Equation allows you to calculate the ∆G, and verify reaction spontaneity, at other Temperatures. GHS Honors Chem Gibbs Free Energy & Spontaneity 1. C3H6(l) + O2(g) Æ CO2(g) + H2O(g) 2. • Using the standard enthalpies and entropies of formation, determine whether the reaction is spontaneous at -20oC. • Balance first …. GHS Honors Chem 3. GHS Honors Chem If ∆G is negative, the forward reaction is spontaneous. If ∆G is 0, the system is at equilibrium. If ∆G is positive, the reaction is spontaneous in the reverse direction. 5 Gibbs Free Energy Worksheet & Free Energy Practice Problems GHS Honors Chem 6