Atomic Term Symbols and Energy Splitting
Chemistry 362
Fall 2015
Dr. Jean M. Standard
November 4, 2015
Atomic Term Symbols and Energy Splitting
1. Atomic Term Symbols and the Sodium D-Line
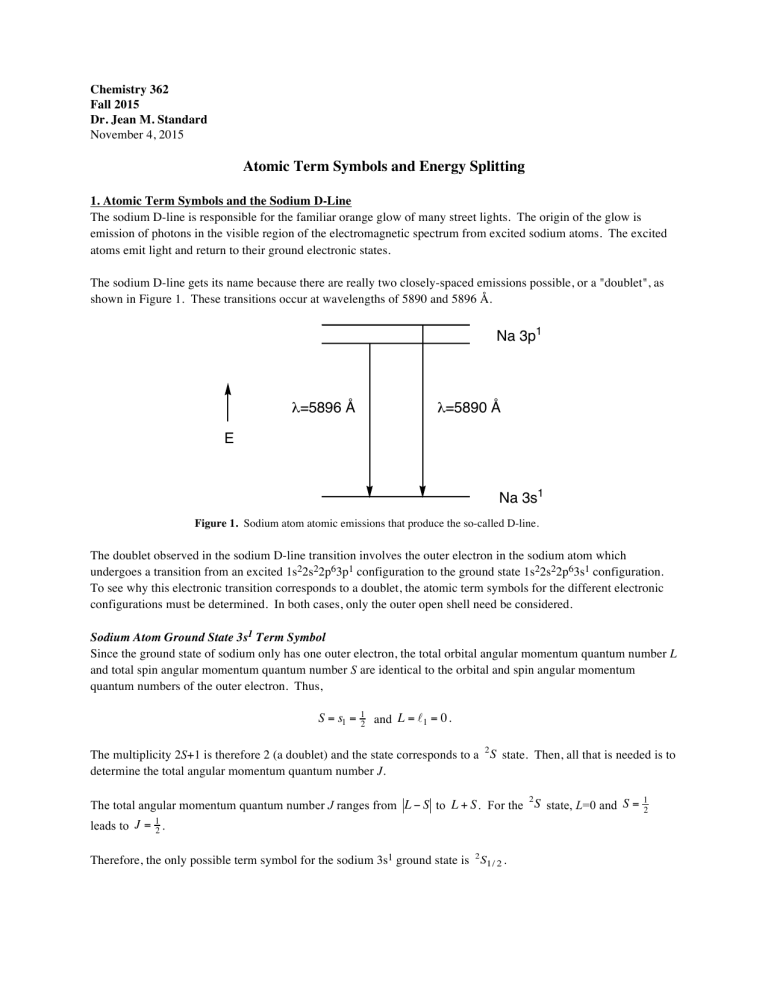
The sodium D-line is responsible for the familiar orange glow of many street lights. The origin of the glow is emission of photons in the visible region of the electromagnetic spectrum from excited sodium atoms. The excited atoms emit light and return to their ground electronic states.
The sodium D-line gets its name because there are really two closely-spaced emissions possible, or a "doublet", as shown in Figure 1. These transitions occur at wavelengths of 5890 and 5896 Å.
Na 3p 1
λ
=5896 Å
λ
=5890 Å
E
Na 3s
1
Figure 1.
Sodium atom atomic emissions that produce the so-called D-line.
The doublet observed in the sodium D-line transition involves the outer electron in the sodium atom which undergoes a transition from an excited 1s
2
2s
2
2p
6
3p
1
configuration to the ground state 1s
2
2s
2
2p
6
3s
1
configuration.
To see why this electronic transition corresponds to a doublet, the atomic term symbols for the different electronic configurations must be determined. In both cases, only the outer open shell need be considered.
Sodium Atom Ground State 3s
1
Term Symbol
Since the ground state of sodium only has one outer electron, the total orbital angular momentum quantum number L and total spin angular momentum quantum number S are identical to the orbital and spin angular momentum quantum numbers of the outer electron. Thus,
S = s
1
=
1
2
and L = ℓ
1
= 0.
The multiplicity 2 S +1 is therefore 2 (a doublet) and the state corresponds to a
2
S state. Then, all that is needed is to
€ €
J .
L + S . For the
2
S state, L =0 and S =
1
2
€
The total angular momentum quantum number J ranges from L
−
S
€
leads to J =
1
2
.
€
1
€
€
S
1/ 2
.
€
€
2
Sodium Atom Excited State 3p1 Term Symbol
The 3p 1 excited state of sodium only has one outer electron, so the total orbital angular momentum quantum number
L and total spin angular momentum quantum number S are identical to the orbital and spin angular momentum quantum numbers of the outer electron. Thus,
S = s
1
=
1
2
and L = ℓ
1
= 1.
The multiplicity 2 S +1 is therefore 2 (a doublet) and the state corresponds to a
2
P state. Then, all that is needed is to
€ €
J .
The total angular momentum quantum number J ranges from leads to two possible values of J , J =
1
2
and J =
3
2
.
L
−
S
€
L + S . For the
2
P state, L =1 and S =
1
2
€
€
1
€
2
P
1/ 2
and
2
P
€
. Spin-orbit
Energy Level Diagram
€ €
€ €
The term symbols determined for the ground and excited states of sodium can be used to label the transitions responsible for the sodium D-line emission, as shown in Figure 2.
Na 2 P
3/2
Na
2
P
1/2
λ
=5896 Å
λ
=5890 Å
E
Na
2
S
1/2
Figure 2.
Atomic term symbols for transitions involved in the sodium D-line.
3
3. Another Example of Energy Splitting of Atomic Terms
Consider an example of an atomic electron configuration 1s
1
2p
1
. There are 12 ways of choosing the individual quantum numbers for the two electrons in this configuration. In the absence of electron-electron repulsions, all these states are degenerate.
The possible term symbols for the 1s
1
2p
1
configuration are
1
P and
3
P (not including the J value). Hund ' s first rule states that terms with higher multiplicity will be lower in energy. Thus, including electron-electron repulsion,
3
P will be lower in energy than
1
P.
For the
1
P term, the only possible value of J is 1; thus, the only term symbol for this state is possible values of J are 0, 1, and 2; this leads to term symbols
3
P
0
,
3
P
1
, and
3
P
2
1
P
1
. For the
3
P term, the
. The total degeneracy of the
3
P terms is 3, 1, 3, and 5, respectively, for a total of 12 (in agreement with the 12 sets of individual quantum
1
P and numbers discussed above).
The
3
P
0
,
3
P
1
, and
3
P
2
states are split in energy by a very small amount. This splitting is due to the coupling of spin angular momentum (S) with total orbital angular momentum (L). This spin-orbit coupling splits levels within the same term (that is, the same values of L and S) that have different values of J.
Finally, if the atom is placed in a magnetic field, the levels with the same values of L, S, and J, but with different values of M
J
are split. All of the energy splittings for the 1s
1
2p
1
electron configuration are summarized in Figure3.
no elec-elec elec-elec spin-orbit
repulsion repulsion coupling
magnetic
field
Figure 3.
Energy splitting of atomic terms in the 1s 1 2p 1 configuration.







