Chemistry 146 Lecture Problems Acetic Acid/Acetate Buffer
advertisement
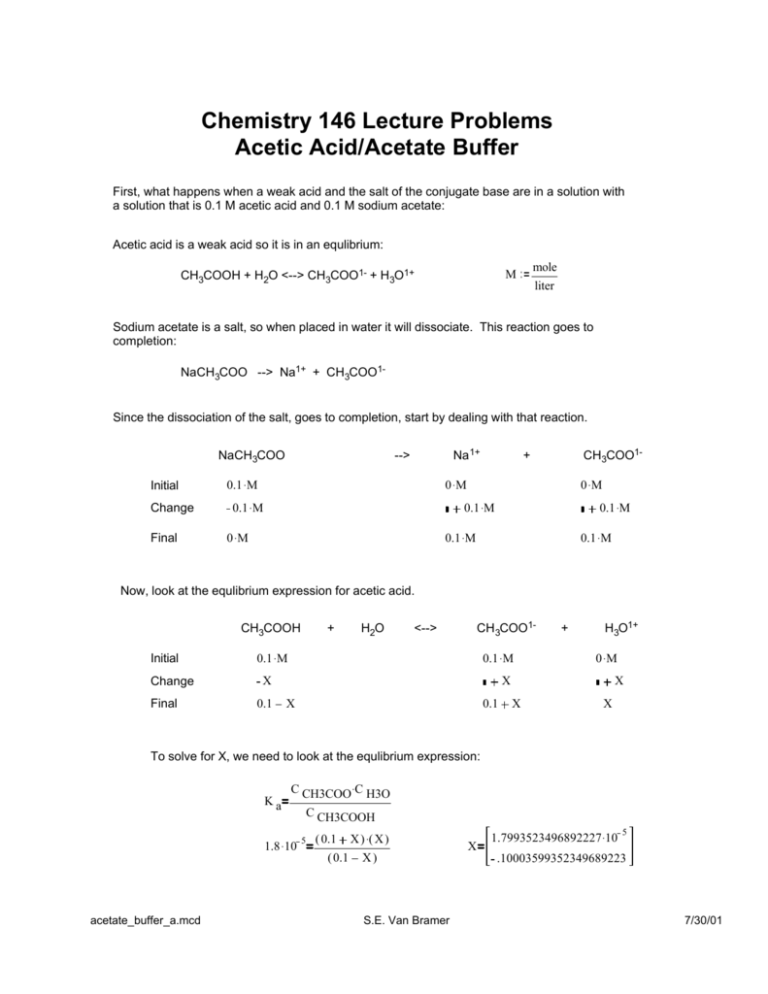
Chemistry 146 Lecture Problems Acetic Acid/Acetate Buffer First, what happens when a weak acid and the salt of the conjugate base are in a solution with a solution that is 0.1 M acetic acid and 0.1 M sodium acetate: Acetic acid is a weak acid so it is in an equlibrium: CH3COOH + H2O <--> CH3COO1- + H3O1+ mole M liter Sodium acetate is a salt, so when placed in water it will dissociate. This reaction goes to completion: NaCH3COO --> Na1+ + CH3COO1- Since the dissociation of the salt, goes to completion, start by dealing with that reaction. NaCH3COO Initial Change Final Na 1+ --> 0.1 . M CH3COO1- + 0 .M 0.1 . M 0 .M 0.1 .M 0 .M 0.1 .M 0.1 . M 0.1 . M Now, look at the equlibrium expression for acetic acid. CH3COOH Initial Change Final + H2O CH 3COO1- <--> 0.1 . M 0.1 . M X 0.1 + H3O1+ 0 .M X X 0.1 X X X To solve for X, we need to look at the equlibrium expression: Ka C CH3COO .C H3O 1.8 . 10 C CH3COOH 5 ( 0.1 ( 0.1 acetate_buffer_a.mcd X ) .( X ) X) S.E. Van Bramer X 1.7993523496892227 .10 5 .10003599352349689223 7/30/01 Simplifying the expression: 1.8 . 10 X ) .( X ) ( 0.1 5 ( 0.1 1.8 . 10 X) ( 0.1 ) .( X ) 5 ( 0.1 ) 1.8 .10 X 5 Essentially the same as the exact solution. Which gives final concentrations for everything as: CH3COOH 0.1 X = 0.09998 CH3COO1- 0.1 X = 0.10002 H 3O1+ X = 1.8 . 10 Notice this value, it is not a coincidence. 5 Finally, look at Kw (Since this is the smallest equlibrium constant): 2 H2O H 3O1+ <---- > 1.8 . 10 Initial Change 5 0 X 1.8 . 10 Final OH1- + X 5 X X Solve for X using the equlibrium expression: K w C H3O .C OH 1.0 . 10 X 1.8 . 10 14 5 X .( X ) 1.8000555538409837511 . 10 5.555384098375108 .10 5 10 Or simplify the expression: 1.0 . 10 X 14 1.8 . 10 5.55556 .10 5 . (X) 10 Which is identical to the exact solution (the meaningful root) Which gives the final equlibrium concentrations as: OH 1- acetate_buffer_a.mcd X = 5.55556 .10 10 S.E. Van Bramer 7/30/01








