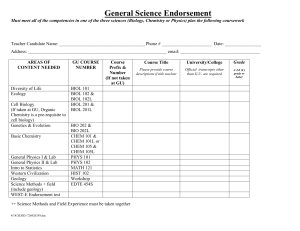
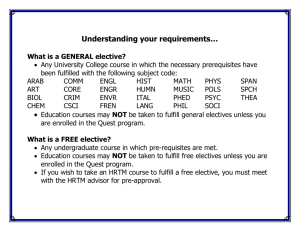
biochemical properties of hormone
advertisement

Annual Reviews www.annualreviews.org/aronline Annu. Rev. Biochem. 1980.49:533-564. Downloaded from arjournals.annualreviews.org by LIBRARY CONTINUATIONS on 01/30/06. For personal use only. Ann.Rev.Biochcm. 1980.49;533-64 Copyright ©1980by Annual Reviews Inc. All rightsreserved BIOCHEMICAL PROPERTIES OF HORMONE-SENSITIVE 1ADENYLATE CYCLASE Elliott 012051 M. Ross Departments of Pharmacologyand Biochemistry, University of Virginia School of Medicine, Charlottesville, Virginia 22908 Alfred G. Gilman Department of Pharmacology, University of Virginia School of Medicine, Charlottesville, Virginia 22908 CONTENTS PERSPECTIVES AND SUMMARY .......................................................................... PROTEINCOMPONENTS OF HORMONE-SENSITIVE ADENYLATE CYCLASE ............................................................................................ Hormone Receptors and.4denylate Cy¢lase ............................................................ Hormone Receptors Linkedto Adenylate Cyclase .................................................. SizeandShape of Adenylate Cy¢lase ...................................................................... Resolution of Catalytic andRegulatory Proteins .................................................... Properties oftheCatalytic Protein (C).................................................................... TheGuanine Nucleotide-Binding Regulatory Protein(G/F).................................. UNC Lesion:Coupling Factoror Covalent Modification ........................................ Calcium-Dependent Regulatory Protein .................................................................. Other Protein Factors .................................................................... ’ ............................ Reconstitution of Hormone-Sensitive Activity.......................................................... 534 535 535 537 538 540 542 543 545 546 547 548 1Abbreviationsused: Gpp(NH)p, guanyl-5’-yl-imidodiphosphate;GTP-~-S,guanosine-5’O-(3-thiotriphosphate);CDR,calcium-dependent regulatory protein (calmodulin);C, catalytic protein of adenylate cyelase; G/F, guaninenucleotide-bindingregulatory protein of adenylatecyclase. Weuse the word"hormone"to represent any hormone,autaeoid, neurotransmitter,or drug that can activate adenylatecyclase in a receptor-mediatedfashion. 533 006-4154/80/0701-0533501.00 Annual Reviews www.annualreviews.org/aronline 534 ROSS & GILMAN EFFECTS OFLIPIDS AND MEMBRANE STRUCTURE .................................... REGULATION OFADENYLATE CYCLASE ACTIVITY .................................. Regulation byGuanine Nucleotides .......................................................................... Catecholaraine-Stiraulated GTPase .......................................................................... ARegulatory GTPase Cycle ...................................................................................... CONCLUSION AND QUESTIONS ............................................................................ Annu. Rev. Biochem. 1980.49:533-564. Downloaded from arjournals.annualreviews.org by LIBRARY CONTINUATIONS on 01/30/06. For personal use only. PERSPECTIVES 549 553 554 555 557 559 AND SUMMARY Adenosine-3’:5’-monophosphate (cyclic AMP)is now recognized as ubiquitous regulatory molecule, controlling diverse metabolic processes in both prokaryotic and eukaryotic organisms. In animals, its principal role is as an intracellular "second messenger"in the transduction of information carried by numeroushormones,and its synthesis is catalyzed almost exclusively by the hormone-sensitive adenylate cyelase system [ATPpyrophosphate-lyase (cyclizing), E.C.4.6.1.1]. Hormone-sensitiveadenylate cyclase activity is found in almost all animal cells (some erythrocytes and cultured cells are exceptions) and, dependingupon the cell, can be stimulated by one or more of a large number of hormones. These include various biogenic amines, proteins, polypeptides, and someprostaglandins. Recently, interest has also focused on negative hormonal control of adenylate cyclase by opiates, c~-adrenergic amines, adenosine, and acetylcholine. Because of the importance of cyclic AMPas a second messenger, interest in adenylate cyclase has centered on the regulation of its activity by hormones and other ligands. As assayed in plasma membranepreparations, adenylate cyclase displays a "basal" activity which varies enormouslyaccording to tissue and assay procedure. It is unclear whether this represents the true activity of the unperturbed enzymeor slight stimulation of an initially inactive enzymeby regulatory ligands (provided as impurities in the membranepreparation or in the ATPused as substrate). This basal activity can be elevated by the addition of appropriate hormones or analogues thereof to the assay mixture, but the extent of hormonalactivation assayed in vitro is generally less than that observed whenthe synthesis of cyclic AMPis studied in intact cells or tissues. The concentration of hormones required to stimulate the enzymeis also frequently increased after homogenization of tissues. Hormonalstimulation of adenylate cyclase also requires the presence of a guanine (or related purine) nucleotide in addition to substrate. This is general requirementfor all cells studied in detail and has led to the finding that various analogues of GTP, such as Gpp(NH)por GTP-T-S,can stimulate adenylate cyclase activity in the absence of hormones,as can GTPitself under someconditions. Fluoride is another ubiquitously stimulatory ligand of eukaryotic adenylat¢ cyclase. Activation usually requires greater than millimolar concentrations of fluoride and is irreversible or only slowly Annual Reviews www.annualreviews.org/aronline Annu. Rev. Biochem. 1980.49:533-564. Downloaded from arjournals.annualreviews.org by LIBRARY CONTINUATIONS on 01/30/06. For personal use only. HORMONE-SENSITIVE ADENYLATECYCLASE 535 reversible. Cholera toxin and several other bacterial toxins also stimulate adenylatc cyclas¢, apparently by catalyzing the covalent modification of one of the components of the enzyme. This review discusses primarily the biochemical basis of these regulatory phenomenaand howthe composition and structure of the system are mirrored in its regulation. The progress of research in this area reflects three challenging properties of the enzyme.First, adenylate cyclase appears to be composed exclusively of intrinsic membraneproteins and depends upon their proper integration in a membranefor hormonal regulation. Thus, while the catalytic, guanine nucleotide-binding, and hormone-bindingproteins may be solubilized with detergents and assayed according to their individual activities, hormonalstimulation of adenylate cyclase is observed onlyin intactmembranes. Second, theproteins of adcnylatc cyclasc arc scarce,and probably noneexistsin a concentration greaterthanI0 pmol/mg membrane protein; somearclO0-fold lessabundant. Third,severaloftheproteins arcquite labile indetergent solution, andfurther apparentlability isprobably caused by theirdissociation during manipulation. Nevertheless, thepastseveral yearshavesccna majorincrease in our understanding of theindividual components of thesystemandthemechanismoftheirinteraction. Itisnowclearthathormone-sensitive adcnylatc cyclasc is composed ofat least threeproteins: a catalytic protein thatis relatively inactive anddisplays noneoftheregulatory properties described above, a guanine nuclcotidc-binding protein thatmediates theaction of the various regulatory ligands, and one or more hormonereceptors. Our understanding of hormonal stimulation of adcnylatc cyclasc suggests that the regulatory protein is the proximal stimulator of the catalyst and that the receptor-hormone complex acts by mediating the binding of pudne nucleotides to a regulatory site. PROTEIN COMPONENTS OF HORMONE-SENSITIVE ADENYLATE CYCLASE HormoneReceptors and Adenylate Cyclase It is nowclear that receptors for hormonesare indeed individual proteins, distinct from adenylate cyclase. This idea derived first from kinetic studies of the activation of adenylate eyclase, particularly in membranesof adipocytes. In these cells multiple hormones,which bind to different receptor sites, seem to competefor a fixed numberof molecules of adenylate cyclase (1, 2). Other arguments for the nonidentity of hormone receptors and enzymehave stemmedfrom observations of their independent ontogenetic regulation (3-6). More direct experimental approaches used chemical genetic manipulation to resolve receptor and enzyme. Schrammshowed Annual Reviews www.annualreviews.org/aronline Annu. Rev. Biochem. 1980.49:533-564. Downloaded from arjournals.annualreviews.org by LIBRARY CONTINUATIONS on 01/30/06. For personal use only. 536 ROSS & GILMAN that N-ethylmaleimide, which was knownto inactivate adenylate cyclase (7, 8), did not destroy the ligand-binding activity of/~-adrenergic receptors of avian erythrocytes (8). Conversely, N,N-dicyclohexylearbodiimide inactivated binding sites for the fl-adrenergic ligand iodohydroxybenzylpindolol at concentrations that did not inhibit the enzyme(9). Using a genetic approach, Insel et al (10) demonstratedthat two clones of cultured cells that are phenotypically deficient in adenylate cyclase (HTCrat hepatomaand an $49 mouselymphomavariant) both retain/3-adrenergie receptors (10). While each does in fact retain one of the two proteins necessary for adenylate cyclase activity, the data clearly showedthat B-adrenergicreceptors are distinct from assayable adenylate cyclase. A more direct demonstration that adenylate cyclase and hormonereceptors are distinct proteins camefrom the cell fusion experiments of Orly, Sehramm,and co-workers (11-14). Sendai virus was used to fuse Friend erythroleukemia cells to turkey erythrocytes that had been treated with N-ethylmaleimide to inactivate adenylate cyelase. The Friend cells have adenylate cyclase but lack B-adrenergic receptors. Plasma membranesfrom the resultant erythrocyte-Friend cell heterokaryons displayed catecholamine-stimulatable adenylate cyclase activity. Cell fusion and membrane preparation were performed in the presence of cycloheximide to prevent de novo protein synthesis, which indicates that the source of stimulation was the interaction of Friend cell enzymewith erythrocyte ~-adrenergic receptor. This receptor-enzyme interaction takes place rapidly (~ 5 min) in the heterokaryon membrane(13). Cell-to-membranefusion rather than cell-tocell fusion can yield similar reconstitution of hormonalstimulation (14, 15), and membranesfrom various cell types containing a variety of receptors have nowbeen used successfully in this procedure. Thus the central postulate of the "floating receptor" modelof the regulation of adenylate cyclase appears to be essentially accurate: hormonereceptors and adenylate cyclase moleculesare discrete proteins, relatively free to diffuse and interact in the plane of the bilayer. Clear physical separation and molecular characterization of adenylate cyclase and its related hormonereceptors were first achieved by Limbird & Lefkowitz (16) and by Haga et al (17). These groups solubilized adenylate cyelase activity and ~-adrenergic receptor sites with reasonably good recoveries, and separated them both by gel exclusion chromatography and sucrose density gradient eentrifugation in detergent solution. A similar strategy was used by Vauquelin et al (18), whoused a $-adrenergic a~nity matrix to separate receptor from enzyme. Separation of other receptors from adenylate cyclase has been less clear cut, presumably for technical reasons. Welton et al (19) showedthat the hepatic glucagon receptor did not exactly cofractionate with adenylate cyclase during gel exclusion chromatography. WhenDufau et al (20) chromatographed detergent ex- Annual Reviews www.annualreviews.org/aronline HORMONE-SENSITIVE ADENYLATECYCLASE 537 tracts of testis and ovary on agarose gel, they foundseveral peaksof receptor binding activity for LH/hCG,one of which coincided with adenylate cyclase. Whetherthis was fortuitous or representative of real association of proteins is unclear, as is the significance of the multiple peaks. Annu. Rev. Biochem. 1980.49:533-564. Downloaded from arjournals.annualreviews.org by LIBRARY CONTINUATIONS on 01/30/06. For personal use only. Hormone Receptors Linked to Adenylate Cyclase The ligand-binding properties of a wide variety of hormonereceptors that act via adenylate cyclase have been studied in the membranes of target cells by the use of appropriate radioactive ligands; a rather large numberof receptors have also been characterized after detergent solubilization. The /~-adrenergic receptor has been partially purified (18, 21), and there is one brief report on the possible purification of a receptor for LH(22). In several cases [ADH(23), LH(24), FSH(25), PTH(26), and B-adrenergic 27, 28)] ligand binding characteristics of the soluble receptor are essentially unaltered from those of the membrane-boundprotein. In the case of the glucagon receptor (19), it has not been possible to bind ligands to the solubilized protein, although a receptoroligand complexhas been solubilized from hepatic membranesthat were first incubated with lzSI-glueagon. This mayreflect a stabilizing effect of ligand uponthe receptor, and the relative instability of the unliganded, detergent-solubilized protein, as was noted to a lesser extent with the receptor for LH(24) and ADH (23). It is interesting that ~-adrenergic receptors solubilized with digitonin (a saponin) can bind agonist or antagonist ligands in solution (16, 27, 28), whereaslinear alkylpolyethyleneoxidedetergents (Lubrol, Brij) have permitted only the solubilization of a receptor-ligand complex(17). Detergent-solubilized preparations of LHand B-adrenergic receptors have been characterized with respect to their hydrodynamicproperties, and both appear to be rather asymmetricproteins that bind fairly large amounts of detergent in solution (Table 1). [The data of Abou-Issa &Reichert (25) on receptors for FSHfrom calf testis, in which no correction for detergent binding was made,are not easily interpretable but are not grossly discrepant from those shown.] Detergent binding to a protein can be taken to indicate the presence of significant hydrophobic surface area, which suggests that these molecules mayinteract with or span the hydrocarbon portion of the plasma membranebilayer (30). However, the amount of detergent bound per receptor molecule is in each case about that found in one micelle [110 molecules of Lubrol PX, 140 molecules of Triton X-100 (30, 31)]. This also consistent with the interaction of a small region of the protein with a single micelle. It is thus possible that these receptors are localized on the outer face of the plasma membrane and interact with the bilayer via a small hydrophobic region, as is the case for cytochrome b5 and cytochrome b5 reductase (32). Annual Reviews www.annualreviews.org/aronline 538 ROSS & GILMAN Table 1 Hydrodynamic properties of hormone receptors Receptor afl-adrenergic Annu. Rev. Biochem. 1980.49:533-564. Downloaded from arjournals.annualreviews.org by LIBRARY CONTINUATIONS on 01/30/06. For personal use only. Stokes radius (~) S2o,w (S) f/fo M r Detergent bound (g/g protein) bGonadotropin 64 3.1 1.8 7.5 x 104 64 6.5 1.6 1.6 × 105 0.7 (Lubrol PX) 0.22 c (Triton X-100) aFrom (17). Source: $49 lymphoma cell. bFrom(24, 29). Sources: rat testis or ovary. CCalculated from (29). Partial specific volumes determined by CsCi gradient centrifugation in presence of Triton X-100. Size and Shape of Adenylate Cyclase While adenylate eyelase was solubilized by nonionic detergents as early as 1962 (33), it has been only recently that careful hydrodynamicstudies the size and shape of the solubilized enzymehave been undertaken. In 1974 Neer (34) used relatively standard methods to determine the sedimentation coefficient and Stokes radius of rat renal adenylate eyelase (Table 2) and calcdated a molecular weight of 159,000 for that enzyme. These studies were performed in the presence of the nonionic detergent Triton X-100 Osoocytylphenoxypolyethyleneoxide), which was used to solubilize the enzyme. Adenylate cyclase displayed an apparent partial specific volumeof 0.74 in these experiments, suggesting that little detergent was boundto it. (This is a typical partial specific volumefor a protein; that of Triton X-100 is 0.94.) Neer argued, therefore, that the renal enzyme has a minimal hydrophobie surface area and probably does not penetrate the lipid bilayer significantly.Bysimilarexperimental approaches, Neer(3 5), Hagaet al (17), and Stengel &Hanoune(36) have shownthat adenylate eyelase from brain, $49 lymphomacells, and liver are larger proteins (Mr "~ 2-3 X 10~). These enzymesalso have larger apparent partial specific volumes, which suggests ¯ significant detergent binding and, hence, a greater relative hydrophobic surface area. In an alternative approach, Schlegd et al (37, 38) used target size theory to estimate the size of rat hepatic adenylate cyclase in intact membranes. Whenthe enzyme was assayed with MgATPas substrate, a target size of 2.3 - 2.4 X 10~ was obtained, in surprisingly goodagreement with hydrodynamicmeasurements of solubilized enzyme. However, nonlinear decay curves suggested the presence of large aggregates within the membranewith sizes similar to those determined by Houslay et al (39). These large targets were hypothesized to represent catalytic protein in association with multimedcregulatory protein. Detailed studies of the size and shape of adenylate cyelase have not been undertaken with enzymefrom manydifferent sources (el Table 2), so that the generality of these measure- Annu. Rev. Biochem. 1980.49:533-564. Downloaded from arjournals.annualreviews.org by LIBRARY CONTINUATIONS on 01/30/06. For personal use only. Annual Reviews www.annualreviews.org/aronline HORMONE-SENSITIVEADENYLATECYCLASE x 8 ~ ~~ z ~=~ 539 Annual Reviews www.annualreviews.org/aronline 540 ROSS & GILMAN Annu. Rev. Biochem. 1980.49:533-564. Downloaded from arjournals.annualreviews.org by LIBRARY CONTINUATIONS on 01/30/06. For personal use only. ments is unknown.However,the interaction of the regulatory and catalytic proteins of the enzymederived from diverse tissues and animals (see below) suggests that most tissues probably have structurally homologousenzymes. Resolution of Catalytic and Regulatory Proteins It had been hypothesized for several years, without muchexperimental support, that hormone-sensitive adenylate cyclase is composedof a regulatory protein in addition to a catalytic protein and hormonereceptors. When the requirement for a guanine nucleotide for hormonal activation was noted, it was natural that the nucleotide binding site was assumedto be on this regulatory protein. The existence of these two distinct proteins was recently demonstrated by two groups. Pfeuffer made use of the atfinity of the regulatory protein for GTPto resolve it from the catalyst (42, 43) while Ross & Gilman took advantage of somatic cell variants that were genetically deficient in one or the other protein (41, 44). Properties. of these proteins are summarizedin Table 3. Pfeuffer (43) found that whena Lubrol PXextract of pigeon erythrocyte membraneswas passed over a columnof .GTP-substituted agarose, stimulation of adenylate cyclase activity in the extract was decreased whenmeasured in the presence of Mg2+ and either NaFor Gpp(NH)p.Elution of the column with either GTPor Gpp(NH)pyielded a fraction that would combine with the unadsorbedfraction to restore activity, but that itself had virtually no adenylate cyclase activity. If elution was performed with GTP, stimulation of the recombined fractions by NaF could be demonstrated, while elution with Gpp(NH)pyielded a reconstituted mixture that was typically activated by that nucleotide. While this separation was not complete (i.e. somebasal and stimulatable activity remainedin the unadsorbed fraction), this was the first concrete argumentfor the involvementof two separate proteins in fluoride- or guanine nucleotide-stimulatable adenylate cyclase activity. Since the affinity chromatographywas based on binding of a regulatory ligand (GTP), and because the residual activity in the unbound fraction had a differentially depleted response to fluoride and Gpp(NH)p, Pfeuffer assumedthat it was the regulatory subunit of the enzymethat was boundto the agarose and that the catalytic protein remained in the unbound fraction. In this same report, Pfeuffer also showedthat solubilization of membranesunder conditions that yielded the resolution described above wouldalso solubilize a 42,000-dalton protein that had been labeled with the photoattinity ligand GTP-~-azidoanilide. Circumstantial data argued strongly that this protein was responsible for the reconstitution of activity in the unboundfraction from the atfinity column(43, 45). Ross & Gilmanresolved the two proteins of adenylate eyclase by virtue of their differing thermal stabilities and their presenceor absencein different clonal cell lines. They had previously shownthat a detergent extract of Annual Reviews www.annualreviews.org/aronline HORMONE-SENSITIVE ADENYLATE CYCLASE 541 Table 3 Protein componentsof hormone-sensitiveadenylate cyclase Annu. Rev. Biochem. 1980.49:533-564. Downloaded from arjournals.annualreviews.org by LIBRARY CONTINUATIONS on 01/30/06. For personal use only. Hormonereceptor Containshormonebinding site on extracellular face Oneor moredifferent receptors per target cell Catalytic Protein (C) 2+ " ATPless than 10%of activity with Mn 2+ ¯ ATP Activity with Mg Not stimulated by hormones,fluoride, or guanine nucleotides Mr = 190,000 Sensitive to mild heating, low concentrationsof sulfhydryl reagents Guaninenucleotide-binding regulatory protein (G/F) Confers uponC ability to use MgATP as substrate Mediatesregulation of C’s activity by fluoride and guaninenucleotides Binds guaninenucleotides and fluoride Probable GTPase Contains 41,000-45,000mol wt cholera toxin substrate Absent in cyc- $49 lymphoma cells Morestable to heat or sulfhydryl reagentsthan is C plasma membranes that contained adenylate cyclase could, under appropriate conditions, recombine with membranes of a phenotypically adenylate cyclase-deficient $49 lymphomacell (denoted cyc-) to yield hormone-sensitive activity (46). 2 The eye- variant ceils retain fl-adrenergic.receptors (10) but lack adenylate cyclase activity assayable in the presence of MgATP, which suggests that the mechanismof the reconstitution may be the interaction of solubilized enzyme with B-adrenergic receptors in or on the eyemembranes. However, thermal denaturation of the soli~ble enzymatic activity at 30° led to only slightly decreased levels of activity in the reconstituted mixture. Thus a heat-inactivated detergent extract of plasma membranes could combine with the inactive eye- $49 membranesto yield relatively high levels of adenylate cyclase activity that could be stimulated by fluoride, Gpp(NH)p, or hormone. Similarly, the heated extract could reconstitute soluble fluoride- or Gpp(NH)p-stimulatable activity upon combination with a detergent extract of eye- membranes. These authors argued that the eyemembranes (or extracts therefrom) were supplying a heat-labile factor intrinsic to adenylate cyclase which was destroyed by the heating of the complementary extract; and that the heated extract was hypothesized to provide a second, more stable component that the eye- cells lacked. Sen2Clonesof eye- variant S49cells wereselectedfromwild-typecells byBourneet al (47). $49 cells are killed by elevatedintracellular concentrationsof cyclic AMP (48). Thereforeagents that activate adenylatecyelasein vivo, such as cholera toxin or fl-adrenergic agonists, may be used for suchselection. Annual Reviews www.annualreviews.org/aronline Annu. Rev. Biochem. 1980.49:533-564. Downloaded from arjournals.annualreviews.org by LIBRARY CONTINUATIONS on 01/30/06. For personal use only. 542 ROSS & GILMAN sitivity to proteases, sulfhydryl reagents, and temperature suggested that both factors were proteins. Ross et al (41) were led to propose that the more heat-labile protein, which is retained in cyc- $49 cells, is the catalytic protein of adenylate cyclase. This suggestion derived from the finding that cyc- membranes(or extracts thereof) contain a Mn2+-dependentadenylate cyclase activity. The protein that displays this Mn2+-dependentactivity has hydrodynamicproperties identical to those of the protein that is active in the reconstitution. The two activities are also similarly thermolabile and are similarly stabilized and labilized by a variety of mixtures of nuclcotides and divalent cations, which suggests that the rcconstitution factor is a Mn2+-dcpcndcnt adcnylate cyclase. It is therefore likely that the Mn2+-dependentactivity is a nonphysiological manifestation of thc catalytic protein of adcnylatc cyclasc. This protein is referred to as C. Fromthese and following studies it is apparent that a minimumof two proteins arc necessary for the expression of physiological adcnylatc cyclasc activity and that a third protein, the receptor, is the site of hormonebinding. If these proteins are not tightly bound to each other, the prospects of purifying "holoadcnylatc cyclasc" maybc bleak. Homeyct al (49, 50) have reported about 5000-fold purification of the cardiac enzymeafter stabilization of the complexwith sodiumfluoride, but this success is nearly unique and has not yet bccn exploited further. Various claims of the purification of adenylatc cyclasc to homogeneity(51, 52) arc not convincing, in part because of the very low specific-activities of the products. Properties of the Catalytic Protein ¯ Little is currently knownabout the physical properties of the catalytic protein (C) of adenylatc cyclase. This paucity of information is duc to the lability and difficulty of preparation of resolved C. The best characterized preparation of C is in a crude Lubrol 12A9extract of the cyc- $49 cell plasma membrane(41, 44). More promising preparations may result from efforts by Pfeuffer to purify C from the unboundfraction in his affinity chromatographic procedure (53) and from Ross’s separation of C from G/F by gel exclusion chromatographyof a somewhatstabilized cholatc extract of hepatic plasma membranes(E. M. Ross, in preparation). Londos ct (54) have also reported the solubilization with deoxycholate of 2+dependentadcnylatc cyclase activity from liver, but the ability to reconstitute activity in the presence of MgATPupon addition of G/F was not explored. Ross ct al (41) reported a molecular weight of C from cyc- $49 cells of 1.9 X 105 (see Table2), and Schlegcl et ai (38) found, by target analysis, that Mn2+-dependent activity from liver has a volumecorresponding to a mass of 1.5 X 105 daltons. Nothing is knownof a possible subunit composition of C. Determination of partial specific volumemakes it seem Annual Reviews www.annualreviews.org/aronline Annu. Rev. Biochem. 1980.49:533-564. Downloaded from arjournals.annualreviews.org by LIBRARY CONTINUATIONS on 01/30/06. For personal use only. HORMONE-SENSITIVE ADENYLATECYCLASE 543 likely that C has a relatively large hydrophobicsurface area, as is consistent with the ability of cholate-solubilized C to be reincorporated into monolamellar phospholipid vesicles upon the removal of detergent (E. M. Ross, in preparation). The MnATP-dependent adenylate cyclase activit~ of C is sensitive to several proteases and sulfhydryl reagents (44). Evidently a second, more reactive, cysteine residue is necessary for the interaction of C with the regulatory protein, since N-ethylmaleimidedestroys the reconstitutive activity of C at a concentration more than tenfold below that which inhibits catalysis (41). C must have a substrate site for ATP,presumably as a divalent cationATPcomplex,but it is not knownif this is the samesite at whichthe effects of nucleotides and metals on stability are exerted. There is at least one regulatory divalent cation-binding site associated with adenylate cyclase (55), but it need not be located on C. There is someevidence for a divalent cation site on the regulatory protein (56). It should be noted that there exist two other Mn2+-dependentadenylate cyclase activities in mammalian tissues, but neither appears to be related to C. Mittal & Murad(57) showedthat guanylate cyclase, upon activation free radicals, can utilize ATPas an alternative substrate and catalyze the formation of cyclic AMP.However, guanylate cyelase from $49 cells is more thermostable than is C (41), and Mn2+-dependentadenylate cyclase activity attributable to C is muchhigher than is guanylate cyclase in the same preparation of eye- membranes.A crude preparation of the guaninenucleotide-binding regulatory protein also failed to confer upon hepatic guanylate cyclase the ability to utilize MgATP as substrate. A soluble Mn2+-dependent, hormone-insensitive adenylate cyclase has also been found in testis (40, 58). However,it does not interact productively with the regulatory protein (41) and is muchsmaller than C (40, 41). Guanine Nucleotide-Binding Regulatory Protein (G/F) Muchmore is knownabout the molecular characteristics of G/F than is knownabout C because of the former’s greater stability and ease of preparation and the ease with which it (or, at least, one of its subunits) can radioactively labeled. Several different preparations of G/F, resolved from C, are nowavailable in different states of purity. The G/F protein of pigeon ery~hrocytes was initially separated from C by affinity chromatographyon GTP-substituted agarose by Pfeuffer (43, 45) and subsequently by Spiegel et al (59). Purification by this procedure (about 60-fold) can be improved upon by subsequent sucrose density gradient centrifugation (43). Mammalian G/F was resolved from plasma membranesof various tissues and cultured cells by the thermal or chemicalinactivation of C and was initially characterized in such crude preparations (41, 44). t3/F from rabbit liver Annual Reviews www.annualreviews.org/aronline Annu. Rev. Biochem. 1980.49:533-564. Downloaded from arjournals.annualreviews.org by LIBRARY CONTINUATIONS on 01/30/06. For personal use only. 544 ROSS & GILMAN plasma membraneshas now been partially purified (perhaps 1000-3000fold over the activity in crude extracts) following solubilization by cholate (J. K. Northup, P. C. Sternweis, and A. G. Gilman, unpublished data). Whilethese techniqu, es denature the activity of the catalytic protein, it is likely that C is physically removedduring the early steps in the preparation. G/F is also found free of C in the plasma membranesof certain clones of HTChepatomacells and in detergent extracts therefrom (41). These cells are phenotypically very similar to the cyc- $49 cell variants, and the existence of these two complementaryclones helps to substantiate the in vivo requirement for both the G/F and C proteins for adenylate cyclase activity. Initial data on the composition of G/F came from the work of Pfeuffer (43), who used [32p]GTP-~-azidoanilide to label pigeon erythrocyte membranes. Of four specifically labeled proteins, the fractionation of a 42,000dalton band on dodecyl sulfate-polyacrylamide gels was consistent with its involvement with adenylate cyclase. Further support for the involvement of a 42,000-dalton protein with G/F function derives from studies using cholera toxin. Strong evidence, reviewed in the previous volume(60), suggests that the crucial step in the stimulation of adenylate cyclase activity by toxin is the ADP-ribosylationof the protein involved with regulation of activity by guanine nucleotides. Using [32p]NADas the substrate for the toxin, both Gill & Meren(61) and Cassel & Pfeuffer (45) were able to label primarily a 42,000-dalton protein in pigeon erythrocyte membranesunder conditions where stimulation of adenylate cyclase is optimal. ADP-ribosylation of this protein has both time and temperature dependence and requirements for GTPand a cytosolic protein that are strikingly similar to those observed for activation of adenylate cyclase (62, 63). The 42,000dalton protein also binds to GTP-agaroseand is eluted in parallel with reconstitutive G/F activity (45). Johnsonet al (64) have similarly labeled wild-type $49 cell plasma membraneswith cholera toxin and [32p]NAD. Johnson et al (65) and Howlett et al (67) demonstrated that G/F, rather than C, was the probable site of action of the toxin. It was therefore of interest that Kaslowet al (64, 66) could label a 45,000-dalton protein membranesof wild-type $49 cells (phenotype +, G/F+), o r HTC cells a nd humanerythrocytes (C-, G/F+) +, but not in the cyc- $49 cell variant (C G/F-), which supports the association of this protein with G/F function. A 45,000-dalton protein in hepatic plasma membranes is also ADPribosylated by cholera toxin, and coelectrophoreses with a major Coomasie blue-stained band in untreated, partially purified preparations of G/F. This protein is also labeled whencholera toxin is used to label eye- membranes that have been previously reconstituted with hepatic G/F (67a). Initial hydrodynamicstudies of native solubilized G/F are not consistent with the 4.2 X 104 - 4.5 X 104 molecular weight obtained in dodecyl sulfate. Howlett et al (56) find varying molecular sizes for G/F from $49 lymphoma Annual Reviews www.annualreviews.org/aronline Annu. Rev. Biochem. 1980.49:533-564. Downloaded from arjournals.annualreviews.org by LIBRARY CONTINUATIONS on 01/30/06. For personal use only. HORMONE-SENSITIVE ADENYLATECYCLASE 545 cells dependinguponthe presence or absence of regulatory ligands. Calculations of Mrrange from 9 X 104 - 1.3 X l05. Pfeuffer (53) has also determined that the S2o, w of G/F varies with ligand present (GTP-’z-Sversus GDP) a range consistent with a monomer-dimerinterconversion. Whether these discrepancies truly represent the formation of a dimer or trimer or the association of dissimilar proteins is uncertain. An intriguing and perhaps related finding by Johnson et al (64) is that cholera toxin catalyzes the ADP-ribosylation of two other proteins in wild-type $49 cell plasma membranes (Mr ~" 5.2 - 5.5 X 104) which are also absent in the cyc- (i.e’. G/F-deficient) variant. Whether these proteins are related to G/F or not is unclear, since humanerythrocytes display G/F activity but lack the larger proteins as detected by cholera toxin-catalyzed ADP-ribosylation (66). The kinetics of the thermal denaturation of G/F activity also led speculation as to its possible subunit structure. It was found that, upon heating at 50° C, the ability of crude G/F to reconstitute Gpp(NH)pstimulated activity decayedabout twice as fast as its ability to reconstitute fluoride-stimulated activity. It was hypothesized that one protein, "F", might restore Mg2+-dependent, fluoride-stimulated activity, and that a second protein, "G", might mediate the guanine nucleotide responses of an "F.C" complex (41, 44). The copurification of these two activities now makesthis idea less appealing, as does the finding that GTPor a mixture of fluoride plus ATPstabilizes both activities. It is likely that G/F represents the binding site for ligands that activate adenylate cyclase in the presence of MgATP. The affinity chromatographic procedure of Pfeuffer and his affinity labelling of a 42,000-dalton protein both imply that G/F contains a binding site for GTP(43, 59). This is also supported by the findings that G/F activity is stabilized by GTPor Gpp(NH)p(41), and that its sedimentation coefficient is decreased in presence of GTP-’/-S or Gpp(NH)p(53, 56). Similarly, fluoride plus 2+) decreases the sedimentation coefficient of divalent cation (Mn2+ or Mg G/F (56), whichsuggests that fluoride also binds to this protein. Effects guanine nucleotides and fluoride upon resolved G/F are generally reversible, in contrast to their irreversible or poorly reversible effects uponintact adenylate cyclase (67). UNC Lesion: Coupling Factor or Covalent Modification Selection of $49 lymphomacells in mediumcontaining/~-adrenergic agonists yields clones of a secondresistant phenotypein addition to cyc-. These cells retain plasma membraneadenylate cyclase activity, which is stimu2+, and the intact cells lated by fluoride or GppfNH)p in the presence of Mg respond to cholera toxin with increased production of cyclic AMP.However, these cells have lost responsivenessboth to/~-adrenergie agonists, the Annual Reviews www.annualreviews.org/aronline Annu. Rev. Biochem. 1980.49:533-564. Downloaded from arjournals.annualreviews.org by LIBRARY CONTINUATIONS on 01/30/06. For personal use only. 546 ROSS & GILMAN selecting agent, and also to prostaglandins E1 and F-,2. Since they retain fl-adrencrgic receptors, as assayed by the binding of [125I]iodohydroxybcnzylpindolol, the lesion appears as an uncoupling of enzymeand receptor-hence the name UNC,for uncoupled phenotype (68). The UNCadenylate cyclase system is thus similar to the adenylate cyclase systems of temporarily refractory astrocytomacells (69, 70), adipocytes of hypothyroidrats (71), and those systems artificially uncoupled by treatment with phospholipases (72), the polyene antibiotic filipin (73-76), or certain other amphiphilic compounds(73). The lesion is not total, since a small response prostaglandins (10% as opposed to tenfold in wild type) is noted, and isoproterenol slightly stimulates the rate of activation of the enzymeby Gpp(NH)p(68). The cyc- lesion (loss of the (3/F protein) and lesion are not complementarywith regard to hormonal stimulation of the enzymeas assayed by reconstitution protocols (15, 46, 77) or in somatic cell hybrids (78). It can be inferred, therefore, that either cyc- is deficient both in G/F and a putative "UNCfactor" or that (3/F in UNCcells is somehow defective. Sternweis & Gilman demonstrated that a crude preparation of (3/F from wild-type $49 cells or rabbit liver can restore responsiveness to hormone to UNCplasma membranes(77), and the ability to reconstitute hormone responses in UNCmembranes cofractionates several thousand fold with (3/F (P. C. Sternweis and A. (3. (3ilman et al, unpublished). UNCplasma membranesare labelled with [32p]NADand cholera toxin, it is also observed that the 45,000-dalton protein characteristic of G/F is shifted to a moreacidic isoelectric point (67a). Theseresults, taken together, provide a strong argument that the UNClesion represents a modification (or lack of required modification) of the G/F protein such that it can longer fulfill its role as a coupling factor betweenreceptor and C. The UNC lesion also abolishes control by guanine nucieotides of the affinity of hormone-bindingto receptor (68). This loss is also restored by rcconstitution with crude G/F (77). It is tempting to speculate that the molecular defect found in the UNCvariant is the site of physiological regulation of G/F function in someof those physiologically uncoupled states mentionedabove (69-71). Calcium-Dependent Regulatory Protein The calcium-dependent regulatory protein (CDR,calmodulin) is an acidic, low-molecular-weight, ubiquitous, Ca2÷-binding protein of mammalian cells. It is recognized as the mediator of Ca2+-dependentcontrol of an increasingly large numberof enzymes(79, 80). In 1975, Brostromet al (81) and Cheunget al (82) showedthat adenylate cyclase in Lubrol extracts cerebral cortex particulate fractions displayed a requirement for CDRfor the stimulatory effects of Ca2+. The requirement in the extracts was demon- Annual Reviews www.annualreviews.org/aronline Annu. Rev. Biochem. 1980.49:533-564. Downloaded from arjournals.annualreviews.org by LIBRARY CONTINUATIONS on 01/30/06. For personal use only. HORMONE-SENSITIVE ADENYLATECYCLASE 547 strated after the chromatographic removal of endogenousCDR.Elution of endogenous CDRby EGTAcan also allow the demonstration of regulation of membrane-boundenzymeby CDR(83-85). There is a wealth of kinetic evidence that CDR.Ca2+ is the active species and that free CDRhas little effect. Brostrom ct al (86) have also demonstrated an enhancement Ca2+ of the hormone-stimulated accumulation of cyclic AMPin C6 glioma cells, which supports the physiological relevance of the action of CDR. CDRhas not, however, been shownto have any effect in preparations from a number of cells whose adenylate cyclase does not normally respond to Ca2+ (86, 87). 2+ is just beginning to be studied The mechanismof the effect of CDR-Ca in detail. Storm and co-workers have recently separated two fractions of brain adenylate cyclase by virtue of the affinity of one fraction for CDRsubstituted agarose (88). They find that the species that does not bind 2+ and is also unresponsive to Gpp(NH)pand unresponsive to CDR-Ca fluoride. Someresponse to these ligands can be restored to this fraction by the addition of a diluted crude membrane extract, and the authors speculate that t3/F is the protein that restores stimulation by t3pp(NH)p,fluoride, 2+ (89). The interaction of CDR-Ca 2+ with G/F is also consisand CDR-Ca 2+ is required tent with the finding by Moss & Vaughan(90) that CDR-Ca for the activation of detergent-solubilized rat brain adenylate cyclase by 2+ activates some adenylate cyclases and not cholera toxin. WhyCDR-Ca others is of great interest and is presumablysubject to analysis by reconstitution protocols. It is unknownwhether unresponsive systems lack the 2+ or whether CDRis so tightly mechanism for interaction with CDR-Ca boundto "unresponsive" cyclase as to be unremovable,as is the case with the phosphorylase kinase-CDR complex (91). The lack of sensitivity unresponsive systems to EGTAor to a wide range of Ca2+ concentrations argues for the former explanation (87). Other Protein Factors During preparation of mammalian plasma membranes, a variable and sometimessubstantial amountof adenylate cyclase activity or responsiveness to activators is lost. Manyinvestigators have noticed amelioration of this loss by resuspension of the membranesin the cytosolic fraction (e.g. 92-95). Mostof these findings probably relate to loss of nucleotides or metal ions. Pecker & Hanoune(92, 93) reported that the stimulatory activity rat liver cytosol was at least 80%sensitive to treatment with phosphatase and was less active than GTP. Katz et al (94) have described a cytosolic fraction of rat liver with similar properties, but have claimed that it was sensitive to proteases and not dialyzable. The absence of chromatographic characterization and the lack of a linear assay makethe data difficult to Annual Reviews www.annualreviews.org/aronline Annu. Rev. Biochem. 1980.49:533-564. Downloaded from arjournals.annualreviews.org by LIBRARY CONTINUATIONS on 01/30/06. For personal use only. 548 ROSS & GILMAN evaluate. A numberof investigators have noted alterations in the activity of adenylate cyclase upon extraction or treatment with detergents and have interpreted them in terms of the removal of protein factors. Bradhamfound that extraction of rat brain membranes with Lubrol PX decreased the responsiveness to fluoride ion of activity that remainedin the pellet, and return of the detergent extract yielded a responsive preparation (96). However, the extract was itself stimulatory after treatment with trypsin or heat and data on .the recovery of total activity are not available. Similar experiments by Cuatrecasas’s group, who used multiply extracted brain membranes and solubilized liver adenylate cyclase, are also unclear (97, 98). Both groups may have removed some G/F, some CDR, or both. It cannot be stressed too often, however,that adenylate cyclase activity and its responsiveness to stimulators are exquisitely sensitive both to detergent concentration and to the ratio of detergent to lipid and protein. This sensitivity can be biphasic (stimulatory and inhibitory) and can be reflected differentially when different activators [e.g. fluoride, Gpp(NH)p]are used. almost arbitrary ratio of activities assayed in the presence of these two ligands can be achieved by manipulation of the concentration of detergents and salts. Hence,it is absolutely essential to monitor total activity and to use appropriate detergent and detergent plus protein controls. Reconstitution of Hormone-Sensitive Activity Reconstitution of hormone-sensitive adenylate cyclase from purified components is an obvious prerequisite to detailed studies of the mechanismof their interaction. Progress toward this goal has so far been limited. Sehramm’s group and others have utilized membranefusion to allow the interaction of proteins present in the membranes of different cells (11-15, 99) (see above). After fusion of complementarycells or of membranesfrom different cells, the relevant proteins apparently diffuse laterally throughout the hybrid membranesuch that they can interact productively. While protocols of this sort have potential as a valuable assay for the presence of receptors, G/F and C, they are limited in that intact membranesmust be used. Anattractive developmentwouldof course be the insertion of solubilized, purified proteins into monolamellarliposomes prior to reconstitution by fusion of different liposomal preparations or of liposomes with membranes. Ross & Gilman (41, 46) have demonstrated that membranesof cyc- $49 lymphomacells, which are deficient in G/F protein, can be reconstituted by the addition of detergent-solubilized G/F to yield hormone-stimulatable activity. The physical mechanismby which the G/F reassociates with the cyc- membraneis obscure and is possibly dependent on the detergent used to solubilize G/F. If G/F solubilized by Lubrol 12A9is mixed with cyc- Annual Reviews www.annualreviews.org/aronline Annu. Rev. Biochem. 1980.49:533-564. Downloaded from arjournals.annualreviews.org by LIBRARY CONTINUATIONS on 01/30/06. For personal use only. HORMONE-SENSITIVE ADENYLATECYCLASE 549 membranes, hormone-stimulated activity can be assayed in the mixture. However,if the mixture is centrifuged, G/F activity remains soluble and can be separated from the apparently unaltered cyc- membranes(46). Stable attachment of Lubrol-solubilized G/F to the cyc- membranesis only observed if the mixture is first incubated with an irreversible activator of the enzyme [Gpp(NH)por a mixture of fluoride plus ATPor ADP]and yields an irreversibly activated enzymethat cannot be stimulated further by hormones(67). If G/F is solubilized with cholate, however,mixing of G/F and cyc- membranesin the presence of MgATP (followed by dilution of cholate and incubation above 4° C) yields stable binding of G/F to the membrane without permanent activation of the enzyme. Sternweis & Gilman(77) have used this protocol to reconstitute what is in most functional respects a wild-type membrane, using either eye- or UNCmembranesas a starting point. The reconstitution of solubilized catalytic protein or of hormonereceptors into either depleted membranesor phospholipid vesicles has proven more ditficult, and there is only one plausible report of the reconstitution of hormone-stimulated activity using totally soluble proteins. Hoffmann (100, 101) has described the reconstitution of dopamine-stimulated adenylate cyclase activity by a cholate-dilution protocol. The starting material is a cholate extract of membranesfrom bovine caudate nucleus mixed with asolectin. Other data suggest that the componentsof the system can also be partially resolved before reconstitution and that substitution of different detergents is feasible (101). This is quite a promising technical advance, since, if it is generallyapplicable, it will allowthe use of increasinglypurified proteins and lipids in reconstituted systems. EFFECTS OF LIPIDS AND MEMBRANE STRUCTURE It is clear that at least someof the protein componentsof the adenylate cyclase system must be bound to an appropriate membranestructure if the enzymeis to respond to hormones. However,the role of the structure and composition of the plasma membranein the regulation of adenylate cyclase activity is one of the most confusing and least carefully documentedareas in the study of this enzyme.The basic observation is that solubilization of the plasma membraneor the addition of any numberof agents that disrupt membranestructure causes a loss of responsiveness to hormone. While solubilizations of active Mg2+-dependentadenylate cyclase, C, G/F, and receptors have been documented, there is to our knowledge no report of soluble, hormone-stimulatableactivity in which both the specificity of the effect of hormoneand the true solubility of the preparation have been demonstrated. The latter criterion has been conspicuously absent from the Annual Reviews www.annualreviews.org/aronline Annu. Rev. Biochem. 1980.49:533-564. Downloaded from arjournals.annualreviews.org by LIBRARY CONTINUATIONS on 01/30/06. For personal use only. 550 ROSS & GILMAN literature until recently; merefailure to pellet activity at 100,000 X g is inadequate. The role of the membraneprobably can be best considered in terms of what a membraneprovides: a permeability barrier and a topologically closed asymmetric surface, a mechanismfor the variable and regulatable association and segregation of molecules within a structured millieu, a hydrophobic environment and a stable hydrophobic-hydrophilic interface, and the opportunity to interact with a variety of specific lipid molecules. Each of these properties has been shownto be important for some enzyme or transport system. There is little evidence to suggest that coupling betweenhormonereceptors and adenylate cyclase is obligately involved with plasma membrane transport or with the existence of a membranepotential, 3 although cyclic AMPitself is involved in the regulation of numeroustransport processes (105). While Moore & Wolff (73) have demonstrated that various ionophores uncouple the TSH-stimulated adenylate cyclase system at lower concentrations than those needed to inhibit enzymatic activity, these concentrations are higher than those needed for ionophoric activity in other membranesystems and the effect possibly reflects disruption of bilayer structure. Wehave also found that a number of proton and cation ionophotos do not prevent stimulation of adcnylatc cyclasc by catecholamines in membranes of wild-type $49 cells at concentrations that arc sufficient to uncouple oxidative phosphorylation (El M. Ross, unpublished). Since the ionophores and such unrelated compoundsas filipin [a polyene antibiotic that can grossly disrupt membranesby interaction with cholesterol (106)], nonionic detergents, cholatc, and phenothiazines all cause qualitatively similar multiphasic effects on adenylate cyclase as a function of increasing concentration (stimulation, uncoupling of hormonalactivation, and inhibition), a primary action on the structure of the bilaycr seemsmorelikely than does an increase in membranepermeability. Arguing for a direct relationship between ion gradients and adcnylatc cyclase, Grollman ct al (107) showedthat thyrotropin causes hyperpolarization of cultured thyroid cells and, in the presence of 50 mMchloride ion, of plasma membranevesicles therefrom. This effect preceded activation of adenylate cyclase by 4-5 min. Although the proton ionophore CCCP(2.5 ktM) abolished the membrane potential, its effect on TSH-stimulatedadenylate cyclase was not reported; any causal relationship between effects of TSHon membranepotential and on adenylate cyclase is thus uncertain. A number of membrane-bound enzymes have been shown to require a specific lipid for activity or to be specifically influenced by one or more 3Thesituation is probablydifferentamong fungiandbacteria(102-104). Annual Reviews www.annualreviews.org/aronline Annu. Rev. Biochem. 1980.49:533-564. Downloaded from arjournals.annualreviews.org by LIBRARY CONTINUATIONS on 01/30/06. For personal use only. HORMONE-SENSITIVE ADENYLATECYCLASE 551 lipids, and such suggestions have been advanced for adenylate cyclase. There exist numerousreports on the stimulatory effects of phospholipids, particularly acidic phospholipids, on preparations of adenylate cyclase that were previously perturbed by detergents, organic solvents, or phospholipases (108-111). Mostof these early findings, including Levey’sprovocative reports on hormone-specific requirements for phospholipids (112, 113), have been neither reproducednor exploited further. In general, no particular effort was madeto promote the reassociation of the lipid (frequently added in bulk form) with the membraneor with soluble adenylate cyclase, nor are there data to indicate whether added lipid interacted with the membraneor merely bound detergent (including endogenous fatty acids, lysophosphatides, etc). The best-documentedstudy of this sort is probably that of Rubalcava &Rodbell (72), whoshowedthat treatment of rat liver plasma membraneswith a phospholipase C with somespecificity for acidic phospholipids abolished stimulation of adenylate cyclase by glucagon without inhibiting fluoride-stimulated activity. Concurrently, the Ka for binding of glucagon was increased and the effect of GTPon glucagon binding was lost (72, 76). Similar results were obtained with a phospholiPase A2. neither ease was there a report of restoration of activity after treatment. An alternative approach to the question of requirements for lipids has been to alter metabolically the phospholipid composition of intact plasma membranes.Engelhard et al (114, 115) grew LMfibroblasts in media supplemented with different ethanolamine/choline analogues. They found a correlation betweenthe degree of substitution of the ethanolaminenitrogen in membranephospholipids and the prostaglandin El-stimulated adenylate cyelase activity. However,when GTPwas added to the assay the effect was muchless striking, which suggests that variable contamination of membranes with endogenous GTPmay have occurred. A variation of the K m for ATPas a function of phospholipid head group was also noted. The data of Hirata et al (116) on effects of the methylation of phosphatidylethanolamine to phosphatidylcholine on hormonal stimulation of the enzyme are intriguing but preliminary. A specific role of cholesterol is even less clear than is that of phospholipids. Klein et al (117) noted a monotonicdecrease in adenylate cyclase activity with increasing amountsof cholesterol in some (but not other) clones of cultured kidney cells, but Hanski& Levitzki (118) noted the opposite effect in turkey erythroeytes. The lateral distribution and association of the protein components of adenylate cyclase maybe crucial to hormonal regulation of adenylate cyclase. The notion of an enzyme(or perhaps free G/F, free C, and G/F plus C) and a receptor "floating" in a fluid mosaicbilayer is probably adequate, but their absolute freedomof motion is unclear. Physically, this motion has not been defined at all. The apparent ability of multiple receptors to corn- Annual Reviews www.annualreviews.org/aronline Annu. Rev. Biochem. 1980.49:533-564. Downloaded from arjournals.annualreviews.org by LIBRARY CONTINUATIONS on 01/30/06. For personal use only. 552 ROSS & GILMAN pete for a pool of adenylate cyclase molecules, as occurs in the multireceptor adipocyte~ system(2), and the ability of receptor from one cell to activate enzyme from another in the membraneof a heterokaryon within minutes of fusion (13) argue for at least relative freedom. On the other hand, Sahyounet al (119) have found that pretreatment of frog erythrocytes with isoproterenol alters the distribution of fl-adrenergic receptors amongsubsequently prepared membranefractions, which suggests some restriction of their lateral distribution. Vargaet al (120) were able to demonstrateclustering of MSHreceptors by labelling Cloudman melanoma cells with 12~I-MSH,either at 0°C or after fixation with paraformaldehyde. Maguire et al (121) have also argued against totally randomdistribution of fl-adrenergic receptors and enzymebased on their apparent stoichiometric relationship in small membranevesicles prepared from $49 cells. The effects of the physical state of the plasma membranebilayer on the adenylate cyclase system are being studied in increasing detail. Several groups have tried to correlate adenylate cyclase activity and its regulation with the physical state of endogenous membranelipids by studying the temperature dependenceof catalysis in the presence of various activators (113, 122-i25). Numerouslinear, upward and downwardcurved, and multiphasic Arrhenius plots have been produced, but few general conclusions can be drawnfrom these studies. Moredirect attempts to relate the fluidity of the bilayer to regulation of adenylate cyclase have involved enrichment of isolated membraneswith different exogenousphospholipids. Houslay ¢t al (126, 127) enriched hepatic plasma membraneswith synthetic phosphatidylcholines and were able to relate, qualitatively, changes in the temperatures of inflections of Arrhenius plots of glucagon-stimulated activity with the thermotropic properties of the lipids used. No inflection was observed when fluoride-stimulated activity was measured. This study was the first to suggest strongly that the state of the bilayer might mediate a ratelimiting process in hormonalactivation. Recently, Bakardjieva et al (128) enriched membranesof Chang liver cells with different phospholipids and comparedadenylate cyclase activity of membraneshigh in dimyristoylphosphatidylcholine (DMPC) with membranes high in dipalmitoylphosphatidylcholine (DPPC)or dioleoylphosphatidylcholine (DOPC).At 37°C, above the gel-liquid crystal transition temperature (T,~) of DMPC and DOPC,the membranesenriched in these lipids had lower adenylate cyclase activity than did control preparations or DPPC-enrichedmembranes. At 17°C, below the Tm of DMPCbut above that of DOPC,the membranes enriched in DMPC or DPPChad roughly equivalent activities that were higher than those of the DOPC-endchedmembranes. It thus appeared as though a relative decrease of activity occurred above Tm. Since the differ- Annual Reviews www.annualreviews.org/aronline Annu. Rev. Biochem. 1980.49:533-564. Downloaded from arjournals.annualreviews.org by LIBRARY CONTINUATIONS on 01/30/06. For personal use only. HORMONE-SENSITIVE ADENYLATECYCLASE 553 ence appeared to be most marked when isoproterenol-stimulated activity was measured, binding to B-adrenergie receptors was measured and found to selectively de~rease at temperatures abovethe T,n of the added lipid. It is not knownwhether the changes in activity and in the numberof assayable fl-adrenergic receptors occur by the same or different mechanisms. A third finding with relevance to the effects of membranefluidity upon adenylate cyclase is that of Levitzki and co-workers, whodemonstratedthat cis-vaccenic acid causes an increase in the rate of activation of turkey erythrocyte adenylate cyclase by Gpp(NH)pplus epinephrine. These experiments were based on the finding of Orly & Schramm(129) that A9-10 Al1-12 cis-monounsaturated fatty acids stimulate adenylate cyclase in these membranes. Levitzki and co-workers (130, 131) found that the increase in the rate constant for activation by Gpp(NH)pplus epinephrine was inversely related to the "microviscosity" of the bilayer, as assayed by the fluorescence anisotropy of 1,6-diphenyl-l,3,5-hexatriene (132, 133). Since Gpp(NH)pdoes not markedly stimulate activity by itself in these membranes,the authors interpreted their results as indicating that collision of receptor and enzyme, controlled by their rates of lateral diffusion and hence by the viscosity of the membrane,was the rate-limiting factor in activation by Gpp(NH)pplus hormone. These experiments do not of course indicate whichmotions are limited, which proteins are involved, or even if the apparent effect of microviscosity is on the motion or conformation of a protein. However,taken together with the studies of Bakardjieva et al (128) and Houslayet al 026, 127), they begin to suggest the major role that the structure of the bilayer plays in the regulation of the system. REGULATION ACTIVITY OF ADENYLATE CYCLASE Most studies of the regulation of adenylate cyclase activity by hormones, nucleotides, and other ligands have been undertaken with a view toward understanding the physiological regulation of cyclic AMPmetabolism in a particular tissue. Consequently, there exists a great mass of data derived from manydifferent cells, frequently obtained using crude homogenates, and only rarely directed toward understanding the primary biochemical mechanismof that regulation. In this section we draw upon only a small numberof studies in an attempt to organize what mechanistic information is available and to correlate it with knowledgeof the individual proteins. Regulation by divalent cations has been discussed elsewhere (55), and still knowfrustratingly little about the molecularactions of fluoride. Hence, Annual Reviews www.annualreviews.org/aronline 554 ROSS & GILMAN Annu. Rev. Biochem. 1980.49:533-564. Downloaded from arjournals.annualreviews.org by LIBRARY CONTINUATIONS on 01/30/06. For personal use only. we will stress the interacting effects of hormonesand guanine nucleotides on the activity and association of G/F and C. Regulation by Guanine Nucleotides Muchof our information on the regulation of hormone-stimulatable adenylate cyclase stems from observations on the effects of guanine nucleotides, first noted by Cryer et al (134) and studied moreextensively by Rodbell and co-workers (135-138). GTP, GDP, or ITP by themselves display diverse inhibitory and sfimulatory effects on adenylate cyclase activity, and these vary with cell type, pH, divalent cation concentration, ionic strength, presence of detergent, and probably a dozen other variables. The key observation, however, is that the presence of some guanine (or related purine) nucleotide is an absolute requirement for the stimulation of adenylate cyclase activity by hormones(136). This requirement has been observed plasma membranesof a number of different cell types (121) but is often difficult to demonstrate due to the presence of contaminating guanine nucleotides in the ATPused as substrate (139) or in the membranepreparation (140), and to the possible ability of ATPitself to serve this function at high concentrations. This effect of guanine nucleotides is also suggested to be significant in vivo by the finding that depletion of intracellular GTPby growth of cells in the presence of mycophcnolicacid decreases the intracellular accumulation of cyclic AMPin response to hormones (141-143). addition to the requirement of guanine nucleotides for hormonalactivation of adenylate cyclase, the concentration of hormonenecessary to stimulate activity is altered by the identity of the nucleotide used in the assay. Thus, isoprotcrenol causes half-maximal stimulation of adenylate cyclase in $49 cell membranesat 100 nMin the presence of GTP, 50 nMin the presence of ITP, 20 nMin the presence of XTF, and 15 nM in the presence of Gpp(NH)p(at t=0; see below) (140). Rodbell and co-workers, using [12~I]iodoglucagon, were also first to observe that those purine nucleotides that permit hormonal activation of adenylate cyclase also frequently decrease the affinity of the receptor for hormone (144). This effect has now been described in some detail for receptors for glucagon (19, 76, 145), prostaglandin l ( 146, 1 47), a B-adrenergic agonists (140, 148-150), and has also been observed with receptors for TSH(73) and FSH(151). The effect is specific for agonist antagonist) ligands of the receptor (148, 149). It should be noted that this interaction of regulatory nucleotides and hormonesis not competitive but rather is a negative heterotropic binding interaction. Thus GTP,ITP, and Gpp(NH)p all decrease the affinity of the receptor for hormonesto an equal maximalextent, although with varying potency (140, 144, 147, 149), and act by increasing the dissociation rate constant for hormone(19, 144, 150). Annual Reviews www.annualreviews.org/aronline Annu. Rev. Biochem. 1980.49:533-564. Downloaded from arjournals.annualreviews.org by LIBRARY CONTINUATIONS on 01/30/06. For personal use only. HORMONE-SENSITIVE ADENYLATECYCLASE 555 It has beeninferred by us and others that the ability of nucleotides to alter binding al~nity for hormonereflects association of receptor with adenylate cyclase [most probably with G/F (41)]. This inference is based on several observations: (a) the effect is lost whenthe enzymeis inactivated with sulfhydryl reagents (O/F must bc inactivated--inactivation of C is insuflicient) (75, 150); (b) it is absent in UNCand cyc- variant S49 cells in G/F is either absent or altered (68, 140); (c) it is restored by reconstitution of UNCor eye- membraneswith crude preparations of G/F (77); (d) it lost whenthe fl-adrenergic receptor and adenylate cyclase are uncoupled by the polyeneantibiotic filipin (74, 75); (e) it is lost uponsolubilization (and hence, uncoupling) of/~-adrenergic receptors (150); and (f) it is only in those fl-adrenergic systems that display highly ctficient coupling between hormonebinding and activation of adenylate cyclase (121). More information on the mechanismof hormonal control came from the use of poorly hydrolyzable analogues of GTP[Gpp(NH)p, Gpp(CH2)p, t3TP-T-S]. Although Gpp(NH)pwas introduced as a substitute for GTP in assays where hydrolysis of nucleotide was likely, it quickly became apparent that it was unique in that Gpp(NH)palmost invariably activated adcnylatc cyclasc in the absence of hormone,the activation was frequently greater than that caused by fluoride or by GTPplus hormone, activation was either irreversible or poorly reversible, and adenylate cyclase was much more stable after activation (42, 138, 152-154). While hormones and Gpp(NH)pmay appear to act synergistically, it was shown by Bennett, Jacobs & Cuatrecasas (155, 156) and others (140, 153) that the effect hormoneis only to increase the rate of activation by Gpp(NH)p rather than to increase the final activity attainable. The intensively studied turkey erythrocyte enzyme, where activation by Gpp(NH)pappears to be totally dependent on hormone, is probably only an extreme case (129, 153). have already stressed that the irreversibility of activation by Gpp(NH)p and the effect of hormonesupon the rate of activation makethe quantitative study of the kinetics of activation by this nucleotide somewhattreacherous (121). There is, in essence, no defined Kact for hormoneunder these conditions unless extrapolation to zero time is made(140), and few have tried quantitate accurately the effects of hormoneon activation kinetics (131, 140, 157-159). Furthermore, any calculation of an incremental activity due to hormoneis a function of time. Manymisleading conclusions have been generated by the application of equilibrium assumptions to the analysis of data gathered using Gpp(NH)p. Catecholamine-Stirnulated GTPase In spite of experimental pitfalls, the data obtained with Gpp(NH)p, Gpp(CH2)p,and ITP led a numberof investigators to speculate on the role Annual Reviews www.annualreviews.org/aronline Annu. Rev. Biochem. 1980.49:533-564. Downloaded from arjournals.annualreviews.org by LIBRARY CONTINUATIONS on 01/30/06. For personal use only. 556 ROSS & GILMAN of either GTPhydrolysis (140, 160, 166) and/or phosphotransferase reactions (161) as a mechanismof regulation of adenylate cyclase. Moreimportantly, it led Cassel & Selinger (162) to discover a catecholamine-stimulated GTPaseactivity in turkey erythrocyte membranesand to relate this activity to the regulation of adenylate cyclase. The specific activity of the catecholamine-stimulated GTPase is low (less than 10 pmol min-1 mg-l) and is assayed above a somewhat larger basal activity. App(NH)pmust be included in the assay to inhibit nonspecific nucleoside triphosphatases, and even this strategy is insufficient to assay the enzymein hepatic plasma membranes,where background activity is 50-fold greater than that in turkey erythrocyte membranes. Nevertheless, these authors showed that the pharmacological specificity, Kmfor GTP, and inhibition by either phospholipases, detergent, or GTP-T-Swere all consistent with the involvement of this activity with the hormonalcontrol of adenylate cyclase (162, 163). Perhaps their most provocative finding, however, was that treatment of plasma membraneswith cholera toxin markedly inhibited the catecholamine-stimulated GTPaseactivity without decreasing the "basal" or background level (164). The dependenceof this inhibition on the concentrations of toxin and NAD is similar to that observed for the activation of adenylate cyclase. The decrease in GTPaseactivity is also consistent with the observations of others that cholera toxin alters the regulation of adenylate cyclase by GTPso as to make GTPappear similar to the poorly hydrolyzablc Gpp(NH)p;i.e. treatment with toxin causes activation by GTPto be greater and longer lasting, increases the potency of hormonein the presence of GTP, and decreases the incremental activity caused by hormones (140, 165-168). These data led Cassel &Selinger to propose an explicit model for the regulation of adenylate cyclase by hormones andguaninenucleotidesbased upon the binding and hydrolysis of GTP(164, 165); the model was quite similar to the proposal of Levinson &Blume (166). Drawingheavily upon knowledgeof the interaction of the prokaryotic translational elongation factors Tu and Ts (169), Cassel & Selinger proposed a regulatory GTPase cycle in which GTP-ligandedadenylate cyclas¢ was the active species and hydrolysis of GTPto GDPwas the primary mechanism of inactivation. Fractional activation of adenylate cyclase wouldthus be proportional to the steady-state concentration of the GTP-liganded state. According to the model, inhibition of GTPhydrolysis by cholera toxin would sustain activation, as would the binding of a nonhydrolyzable GTPanalogue such as Gpp(NH)p. Conversely, hormone was proposed to promote binding GTP, thereby stimulating both GTPaseactivity and adenylate cyclase activity. This facilitation of GTPbinding was suggested to represent the "opening" of the binding site such that GDPcould dissociate and GTP could bind. Using this model, Cassel & Selinger have been able to relate Annual Reviews www.annualreviews.org/aronline Annu. Rev. Biochem. 1980.49:533-564. Downloaded from arjournals.annualreviews.org by LIBRARY CONTINUATIONS on 01/30/06. For personal use only. HORMONE-SENSITIVE ADENYLATECYCLASE 557 activation and inactivation rate constants for adenylate cyclase to steadystate activities of the enzyme(165, 170). As expected from their model, cholera toxin decreases the inactivation rate constant without altering the rate of activation by GTPplus hormone(165), and membraneslabeled with [3H]GTPcould be shown to release [3H]t3DP upon the addition of hormone (171). Simultaneous addition of Gpp(NH)pplus hormone to membranesled to the concomitant release of [3H]GDPand reactivation of enzyme (172). At this point, it should be mentioned that hormone-stimulated GTPase activity still has been assayed only in turkey erythrocytes and only with /~-adrenergic agonists. No rigorous proof of the coupling of GTPhydrolysis to the inactivation of adenylate cyclase exists. Such proof will probably be available only whenthe activities of purified G/F, C, and receptors can be studied in properly reconstituted systems. The supporting evidence is striking, however, and the concept of a regulatory GTPasecycle has provided a motivating and organizing hypothesis for the analysis of the regulation of adenylate cyclase; such a paradigmhas been absent for twenty years. A Regulatory GTPase Cycle The elegant studies by Cassel & Selinger and data from other laboratories can nowform the basis of a muchmore detailed description of the regulatory GTPasecycle. In this section we will try to relate the steady-state’ and kinetic data on the binding of regulatory ligands to their possible effects on the activities and interactions of the proteins discussed in earlier sections. Wewill stress, as a unifying concept, the positive and negative effects of the binding of one ligand to a componentof the adenylate cyclase system upon the binding of a second ligand or protein and the effects of such binding upon enzymatic activity. A schematic diagram of the proposed cycle is shown in Figure 1. The central features are those suggested by Cassel &S elinger (165) and Levinson & Blume(166). Details are as follows: 1. It is assumedthat G/F is the only site of binding of GTPand is the site of GTPhydrolysis. Rodbell and co-workers (173) have proposed multiple binding sites for GTP,but their argumentsdo not seem to us compelling whenit is realized that free G/F and G/F bound to receptors (R) or to can have strikingly different properties. It is assumed G/F-GTPis the active species and is the proximal and sole stimulator of the adenylate cyclase activity of C. (G/F-GTP-R-His presumably too short-lived to be significant.) At present, there are few data to suggest whether GTPpromotes the association of G/F and C (i.e. that the binding of C and guanine nucleotides to G/F is positively cooperative) or whether the nucleotide merely activates a preexistent G/F-Ccomplex. Pfeuffer’s demonstration of an increased sedimentation coefficient of G/F in the presence of both active Annual Reviews www.annualreviews.org/aronline 558 ROSS & GILMAN Annu. Rev. Biochem. 1980.49:533-564. Downloaded from arjournals.annualreviews.org by LIBRARY CONTINUATIONS on 01/30/06. For personal use only. _ ~/ ’,~ t; ~"--#GIF’GTP’- kI G/F-GTP.R’H "- GIF’GDP G/F’GDP-R.H G/F.N.N/k5 Figure ] A hypothetical regulatory GTPase cycle in which hormone-receptor complex (H.R)acts bycatalizingthe displacement of GDP fromG/Fby means of negativeheterotropic binding. C and GTP-T-$suggests that GTP-~-Spromotes stable binding of 12 to O/F (53). Schlegel et al (38) found increases in the apparent size of adenylate cyelase upon treatment with Gpp(NH)p,which also argues for a positive interaction of the binding of (2 and GTPto ~/F. The data of Limbird et al (1"/4) suggest, however, that the G/F-C complex may be relatively stable in the unliganded state. 2. Hydrolysis of GTPby G/F (k 1) causes the inactivation of adenylate cyclase, again as proposed by Cassel & Selinger. C maydissociate at this point. NormallykI is not rate-limiting, but the use of a nonhydrolyzable GTPanalogue or treatment of G/F with cholera toxin decreases kl (165), which increases the concentration of G/F-GTP.It is unknownif free G/F has GTPaseactivity or whether the activity is expressed only when G/F is associated with another protein (e.g. C). In an analogous system, free elongation factor Tu is nearly inactive but becomesan active GTPasewhen bound to the ribosome (169). However,Cassel & Selinger (162) were to inactivate adenylate cyclase with N-ethylmaleimidewithout inhibiting GTPaseactivity. 3, In the absence of hormone,k_2 is the rate-limiting step in the regeneration of G/F-GTP,and k2~ k_2. In turkey erythroeytes, k_2 "~ 0, so that after one cycle of GTPhydrolysis all G/F is in the G/F-GDPform and adenylate cyclase is inactive. Gpp(NH)palone cannot activate adenylate cyclase in turkey erythrocytes because there is no free G/F. In mammalian calls, where Gpp(NH)palone does activate the enzyme, k_2 is assumed be finite but low. In $49 lymphomacell membranes, k_z can be assumed to be at least 0.06 rain-I, the rate constant for activation of adenylateeyclase by Gpp(NH)p (41). Annual Reviews www.annualreviews.org/aronline Annu. Rev. Biochem. 1980.49:533-564. Downloaded from arjournals.annualreviews.org by LIBRARY CONTINUATIONS on 01/30/06. For personal use only. HORMONE-SENSITIVE ADENYLATECYCLASE 559 4. This model differs substantially from that of Cassel &Selinger only in the mechanismby which hormoneis proposed to facilitate regeneration of G/F-GTP, and reflects the suggestion of Blume and co-workers (160, 166) that dissociation of GDPrather than binding of GTPis the regulated step. It is generally compatible with the concept of "collision coupling" suggested by Levitzki and co-workers (158). Wesuggest that the only relevant action of hormoneis the negative heterotropic nature of its binding to G/F-R with respect to guanine nucleotides. By this we mean that creation of a G/F-R-Hcomplexdecreases the affinity of G/F-Rfor nucleotide. Webase this suggestion on the negative heterotropic effect of guanine nucleotides upon hormonebinding, where finite increases in both the Kd (144-150) and the dissociative rate constant (144, 147, 150) for hormone have been documented. From thermodynamic considerations, such an effect must be reciprocal; i.e. if nucleotide increases the Kd for hormone, then hormonemust increase the Kd for nucleotide. This concept is consistent with the demonstration by Cassd & Selinger of the catecholaminestimulated dissociation of [3H]GDP or [3H]Gpp(NH)p from turkey erythrocyte membranes(171, 172); also consistent is the requirement for hormonenoted by Pfeuffer (42, 43) and Spiegel et al (59) to allow exchangeofGDP(a tight ligand) with GMP (a loose ligand) prior to affinity chromatographyof G/F. It should be noted that the reaction path k4" k_5 ¯ k6’k_7 is thermodynamicallyequivalent to the path k_2¯ k3. The receptorcatalyzed path is kinetically muchfaster. Formation of the unstable G/F-RH-GDPcomplex promotes the rapid dissociation (k_5) of what would otherwise be a slowly dissociating (k_2) nueleotide ligand. [See (175) for detailed discussion of the general kinetic implications of negative heterotropic binding.] It can be questioned whysuch diverse effects of purine nueleotides are observed if only one binding protein is involved (173). It is dear that G/F behaves differently when free or whencomplexedwith C. Thus activation of adenylate cyelase is generally irreversible when intact membranesor unfraetionated extracts are treated with Gpp(NH)por fluoride but the effects of these ligands uponfree G/F are reversible (56, 67). Similarly, the reversibility of the effect of Gpp(NI-I)p upon hormonebinding (140), opposedto its irreversible activation of enzyme,can be assumedto reflect action of that nueleotide on C~/F-R as opposed to G/F-C. A number of other discrepancies (173) are similarly explicable. Onemust alwaysconsider whether free G/F, G/F-R, or G/F-Cis the species under study in a particular experiment. CONCLUSIONS AND QUESTIONS As we can describe it at this time, hormone-sensitive adenylate eyelase appears to be composedof at least three interacting proteins. The catalyst Annual Reviews www.annualreviews.org/aronline Annu. Rev. Biochem. 1980.49:533-564. Downloaded from arjournals.annualreviews.org by LIBRARY CONTINUATIONS on 01/30/06. For personal use only. 560 ROSS & GILMAN by itself is relatively inactive, but can be stimulated by a guanine nucleotidebinding protein, G/F, when G/F binds GTP. Upon hydrolysis of GTP, activation is terminated until G/F-GTP is regenerated. This regeneration is catalyzed by the receptor-hormone complex, in that the rate of dissociation of GDPis stimulated by the binding of the hormone-receptor complex to G/F. There is as yet no evidence for the direct interaction of receptor with the catalyst. Evidently, the interaction of G/F with receptor can occur only in a relatively unperturbed membrane, since only then can hormonal stimulation of C be observed. The study of the biochemistry of adenylate cyclase is just beginning. Details are missing and questions proliferate. The kinetics and thermodynamics of the multiple interactions of ligands and proteins are yet to be quantitated. The variability of hormonal responses among different tissues is yet to be related to these basic parameters in any meaningful way. The permissive role of an intact plasma membrane for the interaction of G/F and R has barry been approached. What is the mechanism of action of fluoride on G/F?. What is the nature and significance of the hypothetically modified G/F in the UNCvariant? Beyond this level, biochemical studies of long-term regulation (refractoriness) of adenylate cyelase by hormones have been initiated in several laboratories. Howare the synthesis and stoichiometry of G/F, C, and receptors coordinated? We are just beginning to learn to phrase these questions in a meaningful way. ACKNOWLED(3MENTS We would like to thank many of our colleagues publications. We thank Mrs. Wendy Deaner for the manuscript. Our own studies have been GM26445, AM22125, and NS 10193, and by Grant BC240. for sending us preprints of help in the preparation of supported by USPHSgrants Amerlean Cancer Society Literature Cited I. Perkins, J. P. 1973.Adv.CyclicNucleotide Re~3:1--64 2. Birnbaumer,L., Pohl, S. L., Krans,H. M.J., Rodbell,M. 1970.Adv. Biocher~ PsychopharmacoL 3:185-208 3. Harden,T. K., Wolfe,B. B., Sporn,J. R., Perkins,J. P., Molinoff,P. B. 1977. Brain Re&125:99-108 4. Charness, M.E., Bylund,D. B., Beckman,B. S., Hollenberg,M. D., Snydcr, S. H. 1976. Life ScL 19:243-50 5. Bilezekian, J. P.., Spiegel, A. M., Brown,E. M., Aurbach, G. D. 1977. Mol. Pharmacol.13:775-85 6. Harden,T. K., Foster,S. J., Perkins,J. P. 1979. d. Biol. Chem.254:4416-22 7. Oye, I., Sutherland, E. W.1966. Biochim. Biophys. Acta 127:347-54 8. Schramm,M., Naim, E. 1970. J. BioL Chem.245:3225-31 9. Schramm, M. 1976. J. Cyclic Nucleotide Re~ 2:347-58 10. Insel, P. A., Maguire,M. E., Gilman, A. G., Bourne, H, R., Coffino, P., Melmon,K. L. 1976. MoLPharmacoL 12:1062-69 11. Orly, J., Schramm, M.1976. Proc. NatL Acad. Sci. USA73:4410-14 Annual Reviews www.annualreviews.org/aronline Annu. Rev. Biochem. 1980.49:533-564. Downloaded from arjournals.annualreviews.org by LIBRARY CONTINUATIONS on 01/30/06. For personal use only. HORMONE-SENSITIVE 12. Schramm, M., Orly, J., Eimed, S., Korner, M. 1977. Nature 268:310-13 13. Schulster, D., Orly, J., Seid¢l, G., Schramm, M. 1978. J. Biol. Chert 253:1201-6 14. Schramm, M. 1979. Proc. Natl. Acad. Sci. USA 76:1174-78 15. Schwarzmeier, J. D., Gilman, A. G. 1977. J. Cyclic Nucleotide Res. 3:227-38 16. Limbird, L. E., Lefkowitz, R. J. 1977. Z Biol. Chem. 252:799-801 17. Haga, T., Haga, K., Gilman, A. G. 1977. J. Biol. Chem. 252:5776-82 18. Vauquelin, G., Geynet, P., Hanouae,J., Strosberg, A. D. 1977. Proc. Natl..4cad. Sci. USA 74:3710-14 19. Welton, A. F., Lad, P. M., Newby,A. C., Yamamura, H., Nicosia, S., Rodbell, M. 1977. J. Biol. Chem. 252:5947-50 20. Dufau, M. L., Baukal, A. J., Ryan, D., Catt, K. J. 1977. Mol. Cell. Endocrinol. 6:253-69 21. Caron, M.(~., Srinivasan, Y., Pitha, J., Kociolek, K., Lefkowitz, R. J. 1979. J. Biol. Chem. 254:2923-27 22. Dufau, M. L., Ryan, D. W., Bankal, A. J., Catt, K. J. 1975. J. Biol. Chem. 250:4822-24 23. Roy, C., Rajedson, R., Bockaert, J., Jard, S. 1975. J. Biol. Chem. 250:7885-93 24. Dufau, M. L., Charreau, E. H., Catt, K. J. 1973. J. Biol. Chem. 248:6973-82 25. Abou-Issa, H., Reichert, L. E. Jr. 1977. J. Biol. Chem. 252:4166-74 26. Malbon,C. C., Zull, J. E. 1977. J. Biol. Chem. 252:1079-83 27. Caron, M. G., Lefkowitz, R. J. 1976. J. Biol. Chem. 251:2374-84 28. Limbird, L. E., Lefkowitz, R. J. 1978. Proc. Natl. dcad. Sci. USA 75:228-32 29. Dufau, M. L., Charreau, E. H., Ryan, D., Catt, K. J. 1974. FEBS Lett. 39:149-53 30. Tanford, C., Reynolds, J. A. 1976. Biochim Biophy~ Acta 457:133-70 31. Helenius, A., Simons, K. 1975. Biochim. Biophys. Acta 415:29-79 32. Strittmatter, P., Rogers,M. J., Spatz, L. 1973. J. Biol. Chem. 247:7188-94 33. Sutherland, E. W., Rail, T. W., Menon, T. 1962. J. Biol. Chert 237:1220-27 34. Neer, E. J. 1974. J. Biol. Chem. 249:6527-31 35. Neer, E. J. 1978. J. Biol. Chem. 253:1498-1502 36. Stengel, D., Hanoune,J. 1980. Eur. J. Biochem. 102:21-34 37. Kempner, E. S., Schlegd, W. 1979. Anal. Biochem. 92:2-10 ADENYLATE CYCLASE 561 38. Schlegel, W., Kempner,E. S., Rodbell, M. 1979. Z Biol. Chert 254:5168-76 39. Houslay, M. D., Ellory, J. C., Smith, G. A., Hesketh, T. R., Stein, J. M., Warren, G. B., Metealfe, J. C. 1977. Biochira. Biophy~ Acta 467:208-19 40. Neer, E. J. 1978. J. Biol. Chert 253:5808-12 41. Ross, E. M., Howlett, A. C., Ferguson, K. M., Gilman, A. G. 1978. J. Biol. Chera. 253:6401-12 42. Pfeuffer, T., Helmreich, E. J. M. 1975. Z Biol. Chem. 250:867-76 43. Pfeuffer, T. 1977. J. Biol. Chem. 252:7224-34 44. Ross, E. M., Gilman, A. G. 1977. Z Biol. Chera. 252:6966-6970 45. Cassd, D., Pfeuffer, T. 1978. Proc. Natl. Acad. ScL USA 75:2669-73 46. Ross, E. M., Gilman, A. G. 1977. Proc. NatL dcad. Sci. USA 74:3715-19 47. Bourne, H. R., Coffmo, P., Tomkins, G. M. 1975. Science 187:750-52 48. Daniel, V., Litwack, G., Tomkins, G. M. 1973. Proc. NatL Acad. ScL USA 70:76-79 49. Homey,C. J., Wrenn, S. M., Haber, E. 1977. J. Biol. Chert 252:8957--64 50. Homey,C. J., Wrenn, S. M., Haber, E. 1978. Proc. Natl. dcad. Sc£ USA 75:59-63 51. Stellwagen, E., Baker, B. 1976. Nature 261:719-20 52. Swisloeki, N. I., Magnuson,T., Tierney, J. 1977. Arch. Biochem. Biophyz 179:157-65 53. Pfeuffer, T. 1979. FEBS Lett. 101:85-89 54. Londos, C., Lad, P. M., Nielsen, T. B., Rodbell, M. 1979. J. Supramol. Struct. 10:31-37 55. Garbers, D. L., Johnson, R. A. 1975. J. Biol. Chem. 250:8449-56 56. Howlett, A. C., Gilman, A. G. 1980. J. Biol. Chem. In press 57. Mittal, C. K., Murad,F. 1977. J. Biol. Chert 252:3136-40 58. Braun, R. F. 1975. Proc. Natl. Acad. T., Sc£ Dods, USA 72:1097-1 59. Spiegel, A. M., Downs,R. W. Jr., Aurbach, G. D. 1979. Z Cyclic Nucleotide ges, 5:3-17 60. Moss, J., Vaughan, M. 1979. Ann. Bey. Biochert 48:581-600 61. Acad. Gill, D.Sc£ M.,USA Meren, R. 1978. Proc. Natl. 75:3050-54 62. Enomoto, K., Gill, D. M. 1979. J. Supramol. StrucL 10:51-60 63. Enomoto,K., Gill, D. M. 1980. J. Biol. Chem. 255:1252-58 64. Johnson, G. L., Kaslow, H. R., Bourne, H. R. 1978. J. Biol. Chert 253:7120-23 Annual Reviews www.annualreviews.org/aronline Annu. Rev. Biochem. 1980.49:533-564. Downloaded from arjournals.annualreviews.org by LIBRARY CONTINUATIONS on 01/30/06. For personal use only. 562 ROSS & GILMAN 65. Johnson, G. L., Kaslow, H. R., Bourne, H. R. 1978. Proc. NatL Acad. ScL USA 75:3113-17 66. Kaslow, H. R., Farfel, Z., Johnson, G. L., Bourne, H. R. 1979. Mol. PharmacoL 15:472-83 67. Howlett, A. C., Sternweis, P. C., Macik, B. A., VanArsdalc, P. M., Gilman, A. G. 1979. d. Biol. Chem. 254:2287-95 67a. Schliefer, L. S., Garrison, J. C., Sternweis, P. C., Northrup, J. 1980. d. Biol. Chem. In press 68. Haga, T., Ross, E. M., Anderson,H. J., Gilman, A. G. 1977. Prec. NatL Acad. Sc~ USA 74:2016-20 69. Harden, T. K., Su, Y.-F., Perkins, J. P. 1979. d. Cyclic Nucleotide Re& 5:99106. 70. Su, Y.-F., Harden, T. K., Perkins, J. P. 1979. J. Biol. Chem. 254:38-41 71. Malbon, C. C., Moreno, F. J., Cabelli, R. J., Fain, J. N. 1978. d. Biol. Chem. 253:671-78 72. Rubalcava, B., RodbeH, M. 1973. d. Biol. Chem. 248:3831-37 73. Moore, W. V., Wolff, J. 1974. J. Biol. Chem. 249:6255-63 74. Limbird, L. E., Lefkowitz, R. J. 1976. Mol. Pharmacol. 12:559-67 75. Howlett, A. C., VanArsdale,P. M., Gilman, A. G. 1978. Mol. Pharmacol. 14:531-39 76. Lad, P. M., Preston, M. S., Welton, A. F., Nielsen, T. B., Rodbell, M. 1979. Biochim. Biophy~ Acta 551:368-81 77. J. Sternweis, P. C., Gilman, A. G. 1979. Biol. Chem. 254:3333-40 78. Naya-Vigne, J., Johnson, G. L., Bourne, H. R., Coffmo,P. 1978. Nature 272:720-22 79. Wolff, D. J., Brostrom, C. O. 1979. Adv. Cyclic Nucleotide Rez 11:27-88 80. Cheung, W. Y., Lynch, T. J., Wallace, R. W. 1978. Adv. Cyclic Nucleotide Rez 9:233-51 81. Brostrom,C. O., Huang, Y.-C., Breekenridge, B. M., Wolff, D. J. 1975. Proc. Natl. Acad. ScL USA 72:64-68 82. Cheung, W. Y., Bradham, L. S., Lynch, T. J., Lin, Y. M., Tallant, E. A. 1975. Biochem. Biophyz Rez Commun. 66:1055-62 83. Brostrom, C. O., Brostrom, M. A., Wolff, D. J. 1977. J. Biol. Chera. 252:5677-85 84. Lynch, T. J., Tallant, E. A., Cheung,W. Y. 1977. Arcl~ Biochem. Biophy~ 182:124-33 85. Brostrom, M. A., Brostrom, C. O., Breckenddge, B. M., Wolff, D. J. 1976. 251:4744-50 86. Brostrom, M. A., Brostrom, C. O., Wolff, P. J. 1979. Z Biol. Chem~ 254:7548-57 87. Burgess, W. H., Howlett, A. C., Kretsinger, R. H., Gilman, A. G. 197g. J. Cyclic Nucleotide Re~ 4:175-81 88. Westcott, K. R., LaPorte, D. C., Storm, D. R. 1979. Proc. Natl. Acad. ScL USA 76:204-8 89. Toscano, W. A. Jr., Westcott, K. R., LaPorte, C., Storm, D.76:5582-86 R. 1979. Proc. Natl.D. Acad. Sc~ USA 90. Moss, J., Vaughan,M. 1977. Proc. Natl. Acad. Scl.USA 74:4396-4400 9 I. Cohen, P.,Burchell, A.,Foulke~, J. G., Cohen, P. T. W,, Vanaman, T. C., Nairn, A. C. 1978. FEBS Lett. 92:287-93 92. Pecker, F., Hanoune,J. 1977. J. Biol. Chem. 252:2784-86 93. Pecker, F., Hanoune, J. 1977, FEBS Let~ 83:93-98 94. Katz, M. S., Kelly, T. M., Pifieyro, M. A., Gregerman, R. I. 1978. J. Cyclic Nucleotide Rez 4:389-407 95. Sanders, R. B., Thompson,W. J., Robison, G. A. 1977. Biochim. Biophy&Acta 498:10-20 96. Bradham,L. S. 1977. J. Cyclic Nucleotide Rez 3:119-28 97. Sahyoun, N., Schmitges, C. J., LeVine, H. III. Cuatrecasas, P. 1977. Life 2h1857-63 98. Hebdon, M., LeVine, H. III, Sahyoun, N., Schmitges, C. J., Cuatrecasas, P. 1978. Proc. Natl. Acad. ScL USA 75:3693-97 99. Pike, L. J., Limbird, L. E., Lcfkowitz, R. J. 1979. Nature 280:502-4 100. Hoffmann, F. M, 1979. Z Biol. Chem. 254:255-58 101. Hoffmann, F. M. 1979. Biocher,~ Biophyz Res, Commun. 86:988-94 102. Gonzalez, J. E., Petcrkofsky, A. 1977. Z Supmmol. Struck 6:495-502 103. Peterkofsky, A., Gazdan, C. 1979. Proc. Natl. .4cad. ScL US.4 76:1099-1103 104. Pall, M. L. 1977. £ BIOL Chem. 252:7146-50 105. Strewlcr, G. J., Orloff, J. 1977. Adv. Cyclic Nucleotide Rez 8:311-61 106. De Kruyjff, B., Gerritsen, W. J., Oerle~ roans, A., Demel, R. A., van Deenen, L. L. M. 1974. Blochira. Biophys, Acta 339:30-43 107. Grollman, E. F., Lee, G., Ambesi-Impiombato,F. S., Meldolesi,M. F., Aloj, S. M., Coon, H. G., Kaback, H. R., Kohn, L. D. 1977. Proc. NatL .4cad. Sc£ USA 74:2352-56 108. SuHmovici, S.,Bartoov, B.,Lunenfeld, B. 1975. Biochim. Biophyz Acta 377:454-62 Annual Reviews www.annualreviews.org/aronline Annu. Rev. Biochem. 1980.49:533-564. Downloaded from arjournals.annualreviews.org by LIBRARY CONTINUATIONS on 01/30/06. For personal use only. HORMONE-SENSITIVE 109. Kempcn,H. J. M., DePont, J. J. H. H. M., Bonting, S. L. 1974. Biochim. Biophys, Acta 370:573-84 1,10. Rethy, A., Tomasi, V., Trevisani, A. 1971. Arch. Biochem. Biophyz 147:36-40 111. Pohl, S. L., Krans, H. M. J., Kozyreff, V., Birnbaumer, L., Rodb¢ll, M. 1971. J. Biol. Chert~ 246:4447-54 112. Levey, G. S. 1971. J. Biol. Chem. 246:7405--10 1!3. Levey, G. S. 1971. Biochem. Biophy~ Rex Commu~ 43:108-13 114. Engelhard, V. H., Esko, J. D., Storm, D. R., Sc£ Glaser, M. 1976. Proc. Natl. Acad. USA 73:4482-86 1!5. Engelhard, V. H., Glaser, M., Storm, D. R. 1978. Biochemistry 17:3191-3200 1 i6. Hiram,F., Strittmatter, W. J., Axelrod, J. 1979. Proc. Natl. Acad. ScL USA 76:368-72 117. Klein, I., Moore, L., Pastan, I. 1978. Biochira. Biophy~ Acta 506:42-53 118. Hanski, E., Levitzki, A. 1978. LifeSc£ 22:53-60 119. Sahyoun, N., Hollenberg, M. D., Bennett, V., Cuatreeasas, P. 1977. Proc. Natl. Acad. Sc£ USA 74:2860-64 120. Varga, J. M., Saper, M. A., Lerner, A. B., Fritsch, P. 1976. ,L. Supramo£ Struct. 4:45-49 121. Maguire, M. E., Ross, E. M., Gilraan, A. G. 1977. Adv. Cyclic Nucleotide Re& 8:1-83 122. Kreiner, P. W., Keirns, J. J., Bitensky, M. W. 1973. Proc. Natl. Acad. Sc£ USA 70:1785-1789 123. B~ir, H.-P. 1974. Mol. Pharmacol. 20:597-604 124. Rene, E., Peeker, F., Stengel, D., Hanoune, J. 1978. d. Biol. Chem. 253:838-4l 125. Salmon, C., Dderue-LaBelle, N., Fontaine, Y.-A. 1974. Biochimie 56:1229-38 126. Houslay, M. D., Metealfe, J. C., Warren, G. B., Hesketh, T. R., Smith, G. A. 1976. Biochim. Biophys, Acta 436:489-94 127. Houslay, M. D., Hesketh, T. R., Smith, G. A., Warren, G. B., Metealfe, J. C. 1976. Biochim. Biophy~ Acta 436:495504 128. Bakardjieva, A., Galla, M. J., Helmreich, E. J. M. 1979. Biochemistry 18:3016-23 129. Orly, J., Schramm,M. 1975. Proc. Natl. ,4cad. Sc£ USA 72:3433-37 130. Rimon,G., Hanski, E., Braun, S., Levitzld, A. 1978. Nature 276:394-96 131. Hanski, E., Rimon, G., Levitzld, A. 1979. Biochemistry 18:846-53 ADENYLATE CYCLASE 563 132. Shinitzky, M., Barenholz, Y. 1974. d. Biol. Chem. 249:2652-57 133. Chen, L. A., Dale, R. E., Roth, S., Brand, L. 1977. .L. Biol. Chem. 252:2163-69 134. Cryer, P. E., Jarett, L., Kipnis, D. M. 1969. Biochim. Biophys. Acta 177:586-89 135. Rodbell, M., Lin, M. C., Salomon, Y., Londos, C., Harwood,J. P., Martin, B. R., Rendell, M., Berman, M. 1975. Adv. Cyclic Nucleotide Re~ 5:3-29 136. Rodbdl, M., Birnbanmer, L., Pohl, S. L., Krans, H. M. J. 1971. J. Biol. Chem. 246:1877-82 137. Rodbell, M. 1975. J. Biol. Chem. 250:5826-34 138. Londos, C., Salomon, Y., Lin, M. C., Harwood, J. P., Schramm, M., Wolff, J., Rodbell, M. 1974. Proc. Natl./lcad. ScL USA 71:3087-90 139. Kimura, N., Nagata, N. 1977. J, Biol. Chert~ 252:3829-35 140. Ross, E. M., Maguire,M. E., Sturgill, T. W., Biltonen, R. L., Gilman, A. G. 1977. J. Biol. Chem. 252:5761-75 141. J.. Franklin, T. J.,77:113-17 Twose,P. A. 1977. F~ur. Biochem. 142. Smith, C. M., Henderson, J. F., Baer, H. P. 1977. J. Cyclic Nucleotide Res. 3:347-54 143. Johnson, G. S., Mukku,V. K. 1979. J. Biol. Chem. 254:95-100 144. Rodbell, M., Krans, H. M. J., Pohl, S. L., Birnbaumer,L. 1971. J. Biol. Chem. 246:1872-76 145. Lin, M. C., Nicosia, S., Lad, P. M., Rodbell, M. 1977. Z Biol. Chere~ 252:2790-91 146. Moore, W. V., Wolff, J. 1973. Z Biol. Chem. 248:5705-11 147. Lefkowitz, R. J., Mullikln, D., Wood, C. L., Gore, T. B., Mukherjee, C. 1977. J.. Biol. Chert 252:5295-5303 148. Maguire, M. E., VanArsdale, P. M., Gilman, A. G. 1976. Mol. Pharmacol. 12:335-39 149. Lefkowitz, R. J., Mullikin, D., Caron, M. G. 1976. ,L. Biol. Chem. 251:4686-92 150. d. Williams, L. T.,252:7207-13 Lefkowitz, R. J. 1977. BioL Chem. 151. Abou-Issa, H., Reiehert, L. E. Jr. 1976. J. BioL Chem. 251:3326-37 152. Schramm, M., Rodbell, M. 1975. J. BioL Chem. 250:2232-37 153. Spiegel, A. M., Brown,E. M., Fedak, S. A., Woodard, C. J., Aurbach, G. D. 1976. J. CyclicNucleotideRes. 2:47-56 Spiegel, M.,249:7630-36 Aurbach, G. D. 1974. 154. J. Biol. A. Chem. Annual Reviews www.annualreviews.org/aronline Annu. Rev. Biochem. 1980.49:533-564. Downloaded from arjournals.annualreviews.org by LIBRARY CONTINUATIONS on 01/30/06. For personal use only. 564 ROSS & GILMAN 155. Jaeobs, S., Bennett, V., Cuatreeasas, 1976. J. Cyclic Nucleotide Rex 2:205-23 156. Bennett, V., Cuatreeasas, P. 1976. J. Membr.Bio£ 27:207-32 157. Sevilla, N., Steer, M.L., Levitzki, A, 1976. Biochemistry15:3493-99 158. Tolkovsky,A. M., Levitzki, A. 1978. Biochemistry 17:3795-3810 159. Tolkovsky,A. M., Levitzki, A. 1978. Biochemistry 17:3811-17 160. Blume,A.J., Foster,C. J. 1976.J. Biol. Cher~251:3399-3404 161. Cuatrecasas,P., Jaeobs,S., Bennett,V. 1975. Proc. Nat£ Acad. Sc~ USA 72:1739-43 162.Cassel, D., S¢linger, Z. 1976.Biochin~ Biophys,/lcta 452:538-51 163.Cassel,D., Selinger,Z. 1977.Biochera. Biophys, Rex Commun.77:868-73 164. Cassel, D., Selinger, Z. 1977.Proc.NatL Acad~Sc1 USA74:3307-11 165. Cassel,D., Levkovitz,H., Selinger, Z. 1977. J. Cyclic NucleotideRex 3:393406 166. Levinson,S. L., Blume,A. J. 1977. J. Biol. Chem.252:3766--74 167. Flores, J., Sharp, G. W.G. 1975. J. Clit~ lnvest. 56:1345-49 168. Johnson, G. L., Harden, T. K., Perkins, J. P. 1978. J. Biol. Chem.253: 1465-71 169. Lueas-Lenard,J., Beres, L. 1974. The Enzymes10:53-83 170. Cassel, D., Eckstein, F., Lowe,M., Selinger, Z. 1979. d. Biol. Chem.254: 9835-38 171. Cassel,D., Selinger,Z. 1978.Proc.Natl. Acad. Sc£ USA75:4155-59 172. Cassel, D., Selinger, Z. 1977.J. Cyclic Nucleotide Reg 3:11-22 173. Lad, P. M., Welton,A. F., Rodbell,M. 1977. J. Biol. Chem.252:5942-46 174. Limbird,L. E., Hiekey, A. R., Lefkowitz, R. J. 1979. J. Cyclic Nucleotide Rex 5:251-59 175. Chau, V., Huang,L. C., Romero,G., Biltonen, R. L., Huang,C.-H. 1979. Biochemistry.In press