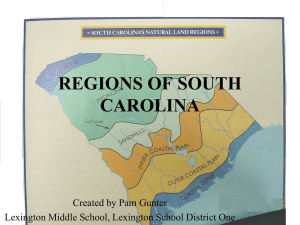
Estuaries of the South Atlantic Coast of North America: Their
advertisement

Estuaries Vol. 23, No. 6, p. 793–819 December 2000 Estuaries of the South Atlantic Coast of North America: Their Geographical Signatures RICHARD DAME1,*, MERRYL ALBER2, DENNIS ALLEN3, MICHAEL MALLIN4, CLAY MONTAGUE5, ALAN LEWITUS3, ALICE CHALMERS2, ROBERT GARDNER3, CRAIG GILMAN1, BJÖRN KJERFVE3, JAY PINCKNEY6, AND NED SMITH7 1 Coastal Carolina University, Conway, South Carolina 29528 University of Georgia, Athens, Georgia 30602 3Baruch Marine Laboratory, University of South Carolina, Georgetown, South Carolina 29442 4University of North Carolina at Wilmington, Wilmington, North Carolina 28403 5University of Florida, Gainesville, Florida 32611 6Texas A&M University, College Station, Texas 77843 7Harbor Branch Oceanographic Institute, Fort Pierce, Florida 34946 2 ABSTRACT: Estuaries of the southeastern Atlantic coastal plain are dominated by shallow meso-tidal bar-built systems interspersed with shallow sounds and both low flow coastal plain and high flow piedmont riverine systems. Three general geographical areas can be discriminated: the sounds of North Carolina; the alternating series of riverine and ocean dominated bar-built systems of South Carolina, Georgia, and northeast Florida, and the subtropical bar-built estuaries of the Florida southeast coast. The regional climate ranges from temperate to subtropical with sea level rise and hurricanes having a major impact on the region’s estuaries because of its low and relatively flat geomorphology. Primary production is highest in the central region. Seagrasses are common in the northern and southern most systems, while intertidal salt marshes composed of Spartina alterniflora reach their greatest extent and productivity in South Carolina and Georgia. Nuisance blooms (cyanobacteria, dinoflagellates, and cryptomonads) occur more frequently in the northern and extreme southern parts of the region. Fishery catches are highest in the North Carolina and Florida areas. Human population growth with its associated urbanization reaches a maximum in Florida and it is thought that the long-term sustainability of the Florida coast for human habitation will be lost within the next 25 years. Tidal flushing appears to play an important role in mitigating anthropogenic inputs in systems of moderate to high tidal range, i.e., the South Carolina and Georgia coasts. The most pressing environmental problems for the estuaries of the southeastern Atlantic coast seem to be nutrient loading and poor land use in North Carolina and high human population density and growth in Florida. The future utilization of these estuarine systems and their services will depend on the development of improved management strategies based on improved data quality. framework related to estuarine type and location. Anthropogenic influences resulting from increasing human population density, urbanization, industrial development, and changing agricultural practices are then addressed. Finally, we synthesize these characteristics into geographical signatures for southeastern estuaries and point out important informational gaps. The paper as a whole seeks to provide a current overview of the status of southeastern Atlantic estuaries and to serve as an informational starting point for those interested in the structure and function of these ecosystems. Introduction The southeastern coast of the United States is a broad coastal plain bordered with barrier islands and beaches interspersed with tidal inlets and rivers. After centuries of relatively low human population density, this highly productive area is now undergoing intense development that is rapidly impacting the structure and function of regional estuarine ecosystems. In this review, we first describe the location and types of estuaries in the region. Then the available published data on the physical parameters, i.e., salinity, water fluxes, and nutrient fluxes, influencing these systems are presented comparatively. The biotic components of these estuaries are examined in a trophic dynamic Geographical and Geological Setting The southeastern coastal plain of the United States is divided into two relatively large geographical reaches. The South Atlantic Bight (SAB) is located between Cape Hatteras, North Carolina (35.38N and 75.58W) and Cape Canaveral, Florida (28.48N and 80.68W) and the southeastern Florida * Corresponding author: address: Marine Science, P.O. Box 261954, Coastal Carolina University, Conway, South Carolina 29528; tele: 843/349-2216; fax: 843/349-2926; e-mail: dame@coastal.edu. Q 2000 Estuarine Research Federation 793 794 R. Dame et al. coast stretches from Cape Canaveral to the Florida Keys (25.58N). This trailing edge coast is distinguished by mainland Pleistocene terraces and Pleistocene or Holocene barrier islands further seaward, and a broad, shallow continental shelf. The ocean boundary is often demarcated by the presence of the Gulf Stream (Florida current south of Cape Canaveral), a major oceanographic current that flows northward along this area transporting heat, materials, and organisms (Fig. 1). The northern portion (the SAB) is a gently curving bight of about 1,000 km (along coast). A band of salt marshes, intersected by networks of tidal creeks, occupies low elevation areas between the mainland and the barrier islands. The salt marshes attain their greatest width (approximately 12 km) at the apex of the SAB between Beaufort, South Carolina and Brunswick, Georgia, where tides for the region reach their maximum range of 3 m. Along its entire extent, the SAB receives input from many rivers of piedmont and coastal plain (blackwater) origin. Two distinct estuarine areas dominate the southeastern Florida portion of the region: the Indian River Lagoon and Biscayne Bay. These systems are also confined to the area between the barrier islands and the mainland, but instead of salt marshes, mangrove wetlands predominate. Indian River lagoon system, including Mosquito Lagoon and Banana River lagoon, stretches north-south from Ponce de Leon Inlet (29.28N), just north of Cape Canaveral, to Jupiter Inlet (26.98N) or about 248 km. Biscayne Bay is bounded to the north by Dumfoundling Bay (25.98N) and to the south by Florida Bay (25.28N). These southeastern Florida estuaries drain the interior of the state east of the northward-flowing St. John’s River and the southwardflowing ‘‘river of grass’’ through the Everglades, via an extensive network of drainage canals and small streams. Without the strong and dedicated service of a cadre of scientists working within the region, it would be difficult to report on the estuaries of the southeast Atlantic coast. For the most part these investigators are associated with university research laboratories, state and federal laboratories, and specific research or protected areas (Fig. 2). While Fig. 2 is not an all-inclusive list, it does show that there is extensive coverage of the estuarine and coastal environments across the region. Types of Estuaries BAR-BUILT AND LAGOONAL Bar-built or lagoonal systems form behind offshore barrier sand islands and usually have small adjacent upland watersheds. Tidal inlets that occur between the barrier islands allow for the exchange of water between the estuaries and the sea. In some cases, the water body behind the barrier islands is extensive and referred to as a sound, i.e., Pamlico Sound, North Carolina. In others, where smaller expanses of water occur behind the islands, the term lagoon is used, (e.g., Mosquito Lagoon, Florida). Throughout the region bar-built systems with extensive marshes and minimal areas of open water are found, (e.g., North Inlet, South Carolina). Astronomical tides are the major forces controlling circulation and water height in inlets, while wind or freshwater runoff is more controlling in sounds. Water flow in the shallow interior portions of lagoons is often controlled by wind action. Because the areas behind the barrier islands are subject to less wave action and are generally depositional environments, their embayments are dominated by extensive wetlands. From North Carolina to Cape Canaveral in Florida, Spartina salt marshes dominate, while further south mangrove swamps prevail. Vernberg et al. (1992) reported that there are more than 320 small bar-built systems (, 60 3 106 m2) between Cape Fear and Cape Canaveral. They also calculated that the total area of these small systems in South Carolina exceeds the total combined area of the major riverine systems, (e.g., Winyah Bay, Santee, Charleston Harbor, and Port Royal Sound). Because these systems are smaller individually than riverine systems, their surface area to volume ratio is higher than that of riverine estuaries. A higher ratio suggests that these small systems may play a greater role in coastal ecological processes than previously thought because they potentially have a greater proportion of surface area over which exchanges of materials can take place. PIEDMONT One type of estuary originates in the hilly areas of the piedmont. These estuaries have extensive watersheds, receive substantial freshwater discharge, usually develop two-layered gravitational circulation in a longitudinally compressed saline zone, may carry suspended sediment loads of clay particles, and have a relatively small proportion of the watershed covered by wetlands. Because high freshwater flow rates (average . 120 m3 s21) carry high loads of suspended clay particles, these systems are sometimes high flow estuaries. The piedmont estuaries common to the region are the Neuse and Cape Fear River in North Carolina, Winyah Bay, Santee, and Edisto systems in South Carolina, and the Savannah and Altamaha of Georgia. Several of these systems are composites of piedmont and coastal plain tributaries. The Cape Fear River originates in the piedmont and is supplemented by two coastal plain streams. Winyah Southeast Atlantic U.S. Estuaries 795 Fig. 1. A sea surface temperature image of the Southeast Coast of the United States (April 14, 1996) showing the Gulf Stream. Cooler waters are in blue and warmer waters are in red. Numbers indicate tidal range in meters and mean monthly minimum temperature in 8C. 796 R. Dame et al. Southeast Atlantic U.S. Estuaries Bay is a complex system where both coastal plain rivers (Sampit, Black, and Waccamaw) and piedmont rivers (Little Pee Dee and Great Pee Dee) mix and enter the Atlantic Ocean through the bay. Also, the Ogeechee estuary in Georgia originates in the piedmont, but most of its flow comes from the coastal plain. COASTAL PLAIN The other type of riverine estuary that occurs in the region is known as a coastal plain system. In this case, the rivers drain watersheds entirely contained within the coastal plain. These systems have sluggish flow rates with low and highly variable discharge, occupy smaller watersheds, possess a larger proportion of wetlands, and generally contain a more extensive saline zone. These systems are often called blackwater rivers because the high concentrations of humic and tannic acids resulting from tree roots and decaying vegetation give their waters a clear black or tea colored appearance. Examples of these estuaries are the New River in North Carolina, the Cooper in South Carolina, Ogeechee, Satilla, and St. Marys in Georgia, and the St. Johns in Florida. FRESHWATER INFLOW Most of the estuaries in the continental U.S. are impacted by human manipulation of their freshwater inflow (Dynesius and Nilsson 1994). In a survey of river fragmentation, 12 of the 19 large rivers (flow . 350 m3 s21) that flow through the continental U.S. were considered strongly impacted, 6 were considered moderately impacted, and 1 (the Pascagoula) was considered unimpacted by channel fragmentation and flow regulation (Dynesius and Nilsson 1994). The survey considered four rivers in the southeast. Two of these, the Santee and the Savannah, were classified as strongly impacted, with 5 dams in each system. The Pee Dee, with 4 dams, and the Altamaha, with 3, were each classified as moderately impacted. The region also includes some of the few remaining unimpounded rivers in the country, and many of the smaller 797 coastal plain rivers (e.g., Waccamaw, Edisto, Ogeechee, Satilla) are free-flowing (Table 1). In addition to the presence of dams and diversions, freshwater, in the form of both groundwater and surface water, is withdrawn from the watersheds of all of the rivers in the region. For example, coastal Georgia has long been dependent on groundwater from the Upper Floridian Aquifer, which underlies all of Florida, coastal Georgia, and parts of eastern Alabama. However, parts of the aquifer are subject to saltwater intrusion (e.g., Brunswick, Savannah, Hilton Head Island) (Clarke et al. 1990). In 1997 the Georgia Environmental Protection Division imposed upper limits on groundwater use in several coastal counties, and in some cases set goals for reductions in groundwater withdrawal (Environmental Protection Division 1997). Traditionally, the water cycle has been presented as water flowing from the land to the sea via rivers and estuaries. Recent evidence from groundwater studies that examined the concentrations of the radioactive isotope 226Ra in the SAB region (Moore 1996) generate estimates of groundwater fluxes to the coastal ocean that are comparable to those of the regions rivers. The magnitude of these fluxes has a number of implications (Church 1996). First, our current estimates of the chemical mass balance in coastal waters may be radically altered. Second, as noted above, increasing human population densities in the coastal zone are making ever greater demands on the coastal aquifers and this results in larger interaction between saline and freshwater aquifers. Further, increasing sea level and the consequential need to maintain navigational channels at even greater depths are also likely to enhance this interaction. As the coastal human population continues to grow, this means that any new or expanded water use in the region will require an alternative water source. In other words, future development along the coast will likely be coupled to surface water withdrawal. Several studies are now underway to understand how increased surface water withdrawal will impact the region’s estuaries. ← Fig. 2. Distribution of estuarine data sources along the southeastern Atlantic coast. KEY: ECU 5 East Carolina University, NEP 5 an Environmental Protection Agency’s National Estuary Program site, DUKE 5 Duke University Marine Laboratory, NMFS 5 National Marine Fisheries Service, UNC 5 University of North Carolina, NCSU 5 North Carolina State University, UNC-W 5 University of North Carolina at Wilmington, NERR 5 National Oceanic and Atmospheric Administration’s National Estuarine Research Reserve, CCU 5 Coastal Carolina University, USC 5 University of South Carolina, BARUCH 5 Baruch Marine Field Laboratory, UCHARLESTON 5 University of Charleston/College of Charleston, SC-DNR 5 South Carolina Division of Natural Resources Laboratory, NOAA 5 National Oceanic and Atmospheric Administration, SKIDAWAY 5 Skidaway Institute of Oceanography, UGA-SAPELO 5 University of Georgia Marine Laboratory at Sapelo Island, LMER 5 a National Science Foundation’s Land-Margin Ecosystem Research, LTER 5 Long Term Ecological Research site, JU 5 Jacksonville University, UFL-WHITNEY 5 University of Florida—Whitney Marine Laboratory, FIT 5 Florida Institute of Technology, HARBOR BRANCH 5 Harbor Branch Institute of Oceanography, UMIAMI-ROSENSTIEL 5 University of Miami—Rosenstiel School of Marine and Atmospheric Sciences. 798 R. Dame et al. TABLE 1. Physical and hydrologic characteristics of southeastern Atlantic coast riverine estuaries. NC 5 North Carolina, VA 5 Virginia, SC 5 South Carolina, GA 5 Georgia, and FL 5 Florida. Drainage Area (km2) Estuarine Zones (km2) State Estuarine Fluvial Tidal Fresh NC, VA NC, VA NC NC SC SC SC SC SC, GA GA GA GA GA FL FL FL 12,585 5,051 5,208 11,176 24,671 1,818 3,089 3,809 3,263 3,752 3,907 8,219 4,386 15,840 3,093 6,746 32,451 5,679 8,859 12,413 22,288 0 38,028 8,454 24,760 8,381 33,055 2,023 0 7,375 0 0 Estuary Albemarle Sound/Chowan/Roanoake Rivers Pamlico/Pungo Rivers Neuse River Cape Fear River Winyah Bay/Pee Dee/Black Rivers North/South Santee Rivers Charleston Harbor/Wando/Cooper/Ashley Rivers St. Helena Sound/S. Edisto/Coosaw Rivers Savannah River Ossabaw Sound/Ogeechee River Altamaha River St. Andrew/St. Simons Sounds/Satilla River St. Marys River/Cumberland Sound St. Johns River Indian River Biscayne Bay Mixing 598 1,899 0 452 5 451 1 76 12 59 0 18 1 58 0 111 7 37 11 39 5 29 7 103 0 7 511 156 0 0 0 94 Seawater AverFW Estuary age Volume Inflow Tidal Area Depth (m3 (m3 Range (km2) (m) 3 109) s21) (m) 0 2,497 0 452 0 456 23 100 17 89 0 18 25 85 92 203 77 121 38 88 5 39 67 176 57 64 17 684 866 866 608 702 4.3 2.7 3.7 3.4 3.0 2.1 4.9 4.0 4.6 4.0 3.4 4.0 6.1 3.4 2.1 2.4 10.7 1.3 1.6 0.3 0.4 0.0 0.6 1.0 0.8 0.4 0.1 0.8 0.5 2.3 1.7 1.6 317 69 118 221 465 366 159 85 344 86 396 65 20 151 34 121 0.6 0.5 0.2 1.1 0.8 1.1 1.4 1.7 2.1 1.9 1.9 2.1 1.8 0.7 0.3 0.5 * Coastal Assessment and Data Synthesis System 1999. SALINITY Understanding the impacts of freshwater input to estuaries and the resulting changes in salinity regimes may be one of the most important challenges facing coastal scientists and managers. Salinity is a primary indicator of estuarine circulation because of its conservative character; it is also a significant determiner of biological productivity, faunal distributions, and habitat structure. Orlando et al. (1994) synthesized salinity data from 15 estuaries in North Carolina, South Carolina, and Georgia by characterizing salinity structure and spatial and temporal variability. A very abbreviated summary of Orlando et al. (1994) findings are provided in Table 2. Three Georgia estuaries, the Altamaha, Satilla, and Ogeechee Rivers are among the most variable in salinity. River input of freshwater is the primary causative agent of salinity variability in these estuaries. Contrastingly, Albemarle Sound, Charleston Harbor, and the Broad River are among the most stable. The latter two systems are shallow coastal plain estuaries with minimal freshwater input, while one of the two rivers that drains into Albemarle Sound is heavily regulated by dams. MATERIAL FLUXES The major materials of carbon, nitrogen, and phosphorus are rapidly translocated, transformed, and remineralized within estuarine ecosystems. Abiotic factors including river flow, tides, and windgenerated waves and currents are major components in translocating materials. Primary production and consumer feeding and metabolism generate both particulate and dissolved materials that influence estuarine nutrient cycles (Dame and Allen 1996). In general, estuaries are large filters or traps (Biggs and Howell 1984) for materials that can be transformed by resident processes. Because of the high productivity of southeastern estuaries, there have been extensive investigations into the fate of materials produced in these systems. In riverine estuaries, transport of dissolved and particulate materials is generally seaward due to the net flux of water down slope due to gravity. Estimates of physical and hydrological parameters for a number of southeastern coastal plain and piedmont riverine estuaries are given in Table 1. The average annual water discharge data clearly distinguishes the two types. To estimate the amount of nutrients being loaded into southeastern riverine estuaries, data on dissolved inorganic nutrient concentrations were compiled from water quality surface sampling stations in 8 of the major riverine systems in the SAB. Water quality data were obtained from EarthInfo (1997), and daily discharge data were obtained from the United States Geological Service (USGS) web site (water.USGS.gov). Only those rivers with systematic sampling programs and consistent analysis protocols were included in this analysis. Stations chosen were located the furthest downstream in each system. In most cases, dissolved inorganic nitrogen (NH4, NO31NO2) records ranged from 1979 through 1994 and dissolved orthophosphate (PO4) records covered from 1981 through 1994. When examined on a quarterly basis, concentrations of ammonium and nitrate plus nitrite did not vary greatly over the course of the year (Fig. 3a,b). However, maximum concentrations were observed Southeast Atlantic U.S. Estuaries 799 TABLE 2. The character of salinity structure and variability in southeastern Atlantic coast estuaries. Data from Orlando et al. (1994). Salinity variability: Very Low 5 ,2‰; Low 5 3–5‰; Medium 6–10‰; High 5 11–20‰. Estuary Albemarle/Pamlico Sounds, North Carolina Bogue Sound (Beaufort, North Carolina) New River, North Carolina Cape Fear River, North Carolina Winyah Bay/North Inlet, South Carolina North and South Santee Rivers, South Carolina Charleston Harbor, South Carolina St. Helena Sound, South Carolina Broad River, South Carolina Savannah River, Georgia Ossabaw Sound/Ogeechee River, Georgia St. Catherines/Sapelo Sounds/Satilla River, Georgia Altamaha River, Georgia St. Andrews/St. Simons Sounds, Georgia St. Marys River/Cumberland Sound, Georgia Dominant Temporal Scale Months-Years Hours-Years Hours-Years Hours-Years Hours-Years Hours-Years Hours-Years, Episodic Hours-Years Hours-Years Hours-Years Hours-Years Hours-Years Hours-Years Hours-Years Hours-Years during the spring quarter (April–June). Orthophosphate concentrations were more variable (Fig. 3c). These were highest during the summer months ( July–September) and lowest during late winter ( January–March). Average annual nutrient concentrations (Fig. 4) were multiplied by average annual discharge or freshwater inflow (Fig. 5) to obtain an estimate of the loads of dissolved inorganic nutrients in each river (Fig. 6). It should be noted that these loads reflect what enters the upper end of the estuaries and not what is loaded to the coastal ocean. Many of these systems have extensive estuarine areas, with additional nutrient sources located downstream of the riverine USGS sampling stations (i.e., point-source discharges, and tidal and non-tidal creeks draining areas of significant human usage). Moreover, the amount of time river water spends in the estuary before being discharged to the coastal ocean will influence the amount of material processing that occurs within the estuary. For example, in the Richmond River estuary in Australia, Eyre and Twigg (1997) found that flushing times controlled whether nutrients were internally processed or discharged to the shelf. In a study of flushing times in the Georgia riverine estuaries, Alber and Sheldon (1999) found that median annual flushing times ranged from 6 d in the Savannah to 72 d in the St. Marys, but that there was considerable interannual and intra-annual variability in these estimates. The highest inorganic nutrient inputs enter into the estuaries of the Cape Fear, Pee Dee, and Savannah Rivers. The Pee Dee and Savannah are the two largest systems considered, with large fluvial drainage basins that likely contribute nutrients in the form of fertilizer runoff. The Cape Fear, although a relatively large river, has about half of the fluvial drainage area and a lower river Mechanism(s) Tides, winds Freshwater, Tides Freshwater, Tides Freshwater, Tides Freshwater, Tides Freshwater, Tides Freshwater, Tides, Winds Freshwater, Tides Freshwater, Tides Freshwater, Tides Freshwater, Tides Freshwater, Tides Freshwater, Tides Freshwater, Tides Freshwater, Tides Variability Low-Medium Medium-High Medium-High Medium Medium Medium Very Low-Low Low-Medium Low Low-Medium Medium-High Medium Medium-High High Low-Medium discharge rate than the Pee Dee and Savannah. However, the highest concentrations of all nutrients were reported in the Cape Fear River, which averaged 0.80 mg l21 N and 0.11 mg l21 P as compared to an average of 0.27 mg l21 N and 0.05 mg l21 P in the remaining 7 rivers. The Cape Fear River basin contains more than one-quarter of North Carolina’s population, and is the most industrialized river basin in the state (641 National Pollution Discharge Evaluation System monitored discharges). Agricultural nutrient sources are also abundant, with approximately 24% of the land usage devoted to agricultural operations (North Carolina Division of Water Quality 1996). The basin also contains over five million head of swine (North Carolina Division of Water Quality 1996), mainly in concentrated animal operations (CAOs) that have only primitive animal waste treatment facilities (Mallin 2000). Recently, Alberts and Takacs (1999) did a similar compilation of USGS water quality data to estimate dissolved organic carbon (DOC) and dissolved organic nitrogen (DON) loads into southeastern U.S. estuaries. They had data from the same rivers used above for inorganic nutrients, with the exception of the Cape Fear and the addition of the Altamaha. DOC concentrations were higher in rivers that originate in the Coastal Plain (average concentrations in the Satilla and St. Marys Rivers were 19.1 and 27.9 mg C l21, respectively) than in those that originate in the Piedmont (average concentrations in the Altamaha and Pee Dee were 8.5 and 5.9 mg C l21, respectively). DON concentrations followed similar patterns, with average concentrations of 0.75 and 0.66 mg N l21 in the Satilla and St. Marys Rivers, respectively, and 0.35 and 0.46 mg N l21 in the Altamaha and Pee Dee, respectively. Although bulk DOC measures generally indicate 800 R. Dame et al. Fig. 4. Average quarterly nutrient concentrations (with SE) for selected coastal plain and piedmont estuaries on the southeast Atlantic coast. simple conservative mixing of freshwater dissolved organic material (DOM) through estuaries, the use of fluorescence signals to trace terrestrial DOM within the Georgia estuaries suggests a highly dy- Fig. 3. Average annual concentration (with SE) of inorganic nutrients in selected coastal plain and piedmont estuaries on the southeast Atlantic coast. Fig. 5. Average discharge of selected coastal plain and piedmont estuaries on the southeast Atlantic coast. Southeast Atlantic U.S. Estuaries Fig. 6. Average nutrient loads in metric tons (with SE) for selected coastal plain and piedmont estuaries on the southeast Atlantic coast. A. ammonium; B. nitrate 1 nitrite; C. orthophosphate. namic picture of the DOM pool, with inputs from freshwater sources, intertidal marsh sources, and even marine sources for some systems (Moran personal communication). The concentrations of particulate material in an estuary can range widely. For example, observed concentrations of suspended sediment in Georgia estuaries ranges from 8–200 mg l21, depending upon sampling depth, tidal stage, and current speed (Georgia Rivers Long Term Ecological Research [GARLMER] data). In the Ogeechee River Estuary, Alber (2000) demonstrated that the biological constituents of the suspended sediment were preferentially associated with fine-grained, 801 slowly settling material, which are likely transported out of the estuary with the net residual flow. Meade (1972) argued that most of the fine-grained sediments that reach the Atlantic Continental Shelf are actually transported back into the estuaries. This contention is supported by an observed rapid decrease in the concentration of inorganic material between the inner and outer shelf, which suggests that river sediment is not being moved seaward (Manheim et al. 1970) and evidence for net landward transport of fine material (Meade 1969; Windom et al. 1971; Mulholland and Olsen 1992). Meade (1972, 1982) further suggested that coastal marshes are important traps for finegrained sediment along the Atlantic coast of North America. This is supported by the observation that marsh elevations are generally keeping up with the relative rate of sea level rise (Vogel et al. 1996), which suggests they are sites of net deposition. However, material that is deposited in marshes may undergo a much longer period of net deposition and resuspension in exchange with tidal creeks (Chalmers et al. 1985) before it is eventually buried (Stevenson et al. 1986, 1988). In a recent study of carbon exchange between the Georgia riverine estuaries and the extensive intertidal marshes that border these systems, Cai et al. (1999) noted that CO2 concentrations measured in estuarine waters could not be explained by respiration within the estuarine water column itself. They demonstrated that CO2 and HCO3 are produced in the marsh, and then funneled into the estuarine water by tidal action. Interestingly, the outwelling of organic matter to the estuaries was minor in comparison to the release of inorganic carbon. Their data show that in a brackish water marsh in the Satilla River Estuary, nitrate that is brought to the marsh via estuarine water is removed, most likely via denitrification (Cai et al. in press). Bar-built and lagoonal systems usually lack significant river flow and have extensive vascular plant subsystems (Kjerfve 1989). In these systems, the transport of particulate material is typically governed by the flux of tidal waters. In the Duplin River, Georgia (Chalmers et al. 1985) and North Inlet, South Carolina (Dame et al. 1986), the tidalvelocity asymmetry, also known as ebb dominance, leads to the seaward transport of suspended materials (Ward 1981; Kjerfve 1989). In some systems, particulate materials may be imported probably as a result of passive and active filtration by subsystems, i.e., salt marshes, oyster reefs, etc. (Dame et al. 1989). In an effort to explain this diversity of material transport, Dame et al. (1992) proposed the geohydrologic continuum theory of marsh-estuarine ecosystem development. In this theory, ma- 802 R. Dame et al. ture components are at the ocean-estuary interface and export particulate and dissolved materials. Young or immature subsystems are at the land-estuary interface and import particulate and dissolved materials. Intermediate systems are midaged and import particulate materials and export dissolved materials. Some bar-built estuaries may have all three developmental stages or subsystems, e.g., North Inlet (Dame et al. 1992), while others may only have one or two subsystems. In lagoonal systems, many materials are processed and retained by the estuary so that only some substances are transported to the adjacent sea. Which materials are processed, retained, or transported depends on the specific subsystem. Tidal marshes, for example, function as giant filters or traps removing and processing large quantities of suspended particulate and dissolved materials from tidal waters and releasing other materials into ebbing tides or during rain events at low tide (Chalmers et al. 1985; Dame 1989). This high retention efficiency is well documented in North Inlet, where more nitrogen and phosphorus are recycled within the marsh than are transported to or from the adjacent water column (Dame et al. 1991). In contrast, Northern Indian River Lagoon has little opportunity for export because there is little tidal flux or river input (Montague and Odum 1997). As sea level rises, the active and passive retention of materials by salt marshes is important in maintaining the size and elevation of the major estuarine subsystems. Vogel et al. (1996) have calculated that the amount of inorganic sediments exported by South Carolina riverine systems is about equal to the amount of this material necessary for salt marsh elevation to keep pace with sea level rise. In an effort to develop regional nitrogen budgets and riverine nitrogen and phosphorus fluxes for drainages to the North Atlantic Ocean, Howarth et al. (1996) estimated discharge and material exports for the southeastern Atlantic coast. Total phosphorus fluxes were estimated to be 0.011 Tg yr21 and total nitrogen was 0.24 Tg yr21or about 32 and 676 kg km22 yr21 respectively. Our loading estimates (Fig. 3), were not for the whole region (we only used rivers with consistent water quality records), and only took into account dissolved inorganic nutrients. They are therefore much less than the totals reported in Howarth et al. (1996). It should be noted that both of these estimates are for material reaching the head of the estuaries only, and so do not take into account either additions or subtractions that may take place as the water moves to the coastal ocean. They do not account for potential inputs from lagoonal systems with little or no river flow, which dominate the southeastern Atlantic coast. At present, it is unclear whether these systems represent net sources or sinks of nutrients to coastal water. Finally, groundwater flow needs to be taken into account, because it can be an additional, direct source of nutrients to the nearshore (Moore 1996). Vogel et al. (1996) computed a first approximation of the relative contribution of tidal water exchange by bar-built and lagoonal systems and discharge by riverine systems in South Carolina. They estimated that tidal water exchange was over an order of magnitude higher than river discharge and that nutrient exports by bar-built systems were at least an order of magnitude greater than riverine delivery to the coastal ocean. If the Howarth et al. (1996) estimates for the southeast Atlantic coast are increased an order of magnitude then this region would be more comparable to the Mississippi Basin of the Gulf Coast in material fluxes and water discharges. Climate The climate of the southeastern coast is Carolinian temperate from Cape Hatteras to north Florida and transitional to subtropical from there to Biscayne Bay. Water temperatures in shallow estuaries, lagoons, and sounds often exceed 308C during July and August and can drop to less than 108C at the northern extremes of the region during February. Seasonality is well defined in the Carolinas and Georgia. Along the south Florida coast there is a pronounced wet period between May and October and a dry period from November to April. The wet-dry season periodicity in south Florida results in an alternation between brackish and hypersaline conditions in many parts of the Indian River Lagoon. HURRICANES The entire region is vulnerable to tropical storms, hurricanes, and tornadoes. Occasionally, tropical storms cross the Gulf Stream and make landfall in the region. During the past century, Florida has been the most hurricane-prone state in the U.S. with 57 strikes (including 24 category 3 or greater) making landfall (National Oceanic and Atmospheric Administration 1997). In the last decade, hurricanes Hugo (1989) near Charleston, South Carolina, Andrew near Miami, Florida (1992), and Bertha and Fran (1996), Bonnie (1998) and Dennis and Floyd (1999) near Wilmington, North Carolina, struck the southeast coast. These storms brought significant winds, waves, flooding, erosion, and associated damage to maritime forests and property. High river discharge associated with rainfall, rerouting of human sewage because of power outages, and extensive animal waste pollution from industrial hog farms Southeast Atlantic U.S. Estuaries in river floodplains combined to produce severe anoxia/hypoxia for weeks following these events (Van Dolah and Anderson 1991; Mallin et al. 1999; Mallin 2000). Within estuaries, hurricanes have been shown to build washover fans on barrier islands, form new tidal inlets by cutting barrier islands, move large quantities of sediments both into and out of estuarine areas, destroy forest, alter adjacent watersheds, and dramatically modify estuarine circulation due to extensive water runoff from rain and storm surges. The impact of a given storm depends on its size, location, speed, the state of the tide, shoreline configuration, and slope of the adjacent continental shelf (Hayes 1978). Besides these factors, the water quality damage associated with hurricanes depends on the amount and type of floodplain development, and whether the hurricane impacts well-flushed systems or poorly-flushed rivers and estuaries (Mallin et al. 1999). For example, in 1989, the eye of Hurricane Hugo struck the South Carolina coast just north of Charleston Harbor estuary and about 75 km south of Winyah Bay and North Inlet estuaries. This Category 4 storm, with sustained winds of 39 m s21 (140 km h21) and a central pressure of 935 mbars, moved onto the coast at a velocity of 12 m s21 at high tide resulting in massive property damage along the coast (Gardner et al. 1992). In Charleston County where there were wind gusts over 200 km h21 and a storm surge of up to 9 m above sea level, Tissue et al. (1994) reported that the storm not only resuspended and winnowed sediments, and eroded channel banks, but also scoured and exported older deposits in large quantities. At North Inlet, the geomorphology of the landscape and the estuary showed little change, although several small new washover fans were formed on the adjacent barrier islands. The nearby maritime forest, however, experienced extensive wind damage, tree mortality, and loss of terrestrial animals in the surge-affected forest immediately after the storm. Major changes in the age structure of the forest and related ecological functions due to this hurricane will be recognized for decades (Gardner et al. 1992). ENSO Inter-annual global climate changes also affect the southeastern Atlantic coast of North America. The best know of these cycles is the El Niño-Southern Oscillation (ENSO) climate event. El Niño and La Niña are terms used to denote opposite phases of this cycle. This climatic cycle occurs every 2 to 7 yr and generally begins in October and ends in March (Ropelewski and Halpert 1986). Individual ENSO events are quite variable in North America 803 and this variability has been attributed to differences in Eastern Pacific sea surface temperatures and internal atmospheric processes in North America. The latter is thought to account for a majority of the variability in the southeastern U.S. (Hoerling and Kumar 1997). ENSO events are thought to influence the southeastern Atlantic coast in a number of ways. First, during El Niño years, winter temperatures are cooler and precipitation may exceed normal values by 3 cm or more (Ropelewski and Halpert 1986; Philander 1990; Hanson and Maul 1991). Although higher than normal freshwater discharge to southeastern estuaries with a higher groundwater table should be expected during these events, these features are only statistically observed from the northeastern coast of South Carolina to Florida (Kuhnel et al. 1990). Second, hurricane frequency in the Atlantic is usually reduced during El Niño years and that implies fewer storm impacts along the southeast coast. During the La Niña phase, the southeastern Atlantic coast experiences warmer winter temperatures with drier conditions when compared to average years. A recent synthesis by Sun and Furbish (1997) found that El Niño and La Niña are responsible for up to 40% of the annual precipitation variation and up to 30% of the river variations in Florida. During the recent 1997–1998 El Niño, few Atlantic hurricanes were observed and rainfall was heavy throughout the region from December through March. Florida and the coastal zone of the Carolinas received over 200% of normal precipitation for this period, while inland areas received 150% of normal precipitation. In North Inlet, a normally euhaline estuary, the increased rainfall depressed salinities below 20 psu for over 3 mo and below 10 psu for one extended period, setting a 20-yr record. SEA LEVEL Sea level is another indicator of global climate change both on seasonal and annual time scales. Mean sea level varies seasonally due to changes in surface pressure (inverse barometer effect) and steric variations, largely the result of heating of estuarine and shallow shelf waters during the summer months and cooling during winter months (Patullo et al. 1955). Measurements of water level at North Inlet, South Carolina indicate that sea level in mid-September on average is 0.25 m higher than mean sea level in February (Kjerfve et al. 1978). As a result, complete flooding of salt marshes occurs most commonly during high tides in September. Similarly, water level in Indian River is 0.27 m higher in October than in most of the rest of the year. This results in a high tide level in winter that is lower than the low tide level in October. 804 R. Dame et al. Long-term water level measurements at Charleston, South Carolina and calculations of mean sea level there indicate that relative sea level has increased 0.25 m since 1922 (3.3 mm yr21), both as a result of eustatic sea level rise (50%) and coastal land subsidence (50%). Recent analyses of cores using the lead-210 technique at North Inlet, South Carolina show a rate of sea level rise of about 3.2 mm yr21 (Vogel et al. 1996) or about the same rate as the Charleston data. Water level records (1913 to present) at Key West show an average rise of 1.7 mm yr21, almost entirely the result of eustatic sea level rise. There is little doubt that sea level is rising along the southeastern Atlantic coast and it will gradually force the transgression of the barrier islands and estuaries of the region upslope along the coastal plain (Dame et al. 1992). These are the same areas where human population density is rapidly increasing with associated increases in buildings and infrastructure. Eventually, sea level rise and human development will compete for the same coastal landscape, but before that significant damage due to storms will likely increase. Primary Producers Primary production is relatively high in southeastern estuaries, and is partitioned amongst a variety of plant and algal communities. A predominant contribution by vascular plants is suggested by their vast aerial coverage over much of the southeastern coast, especially in South Carolina and Georgia. Compared to more northern temperate estuaries, the relative expansiveness of marsh area and high vascular plant productivity in South Carolina and Georgia has been attributed to characteristically high tidal amplitude, low tidal energy, the prevalence of barrier islands, high solar input, a lower seasonal temperature differential, and the absence of snow cover or ice rafting influence (Turner 1976; Haines and Dunn 1985). Measurements of macrophytic primary productivity have generally exceeded algal-based estimates in those southeastern estuaries studied (see Total Primary Production section below), but too few systems have been adequately examined to draw strong general conclusions. Although, in North Carolina and Florida estuaries, research efforts targeting phytoplankton production are somewhat equitable with those examining vascular plant production, our understanding of the relative importance of these groups in South Carolina and Georgia estuaries suffers from a dearth of information on phytoplankton community production (Bricker et al. 1999). Studies on benthic microalgal production are even scarcer, but recently have drawn attention as potentially significant sources of fixed carbon (e.g., Pinckney and Zingmark 1993a; Sig- mon and Cahoon 1997; Cahoon et al. 1999). Even less is known about the relative activity of macroalgae, traditionally considered insignificant to overall estuarine production, but more recently recognized as seasonally important primary producers in some systems (see below). Generally, there are no submerged seagrasses in South Carolina and Georgia because of restricted light penetration. MICROALGAE Microalgal communities from the southeastern area can be broadly classified as phytoplankton or benthic microalgae (which includes periphyton and edaphic algae). Tidal flushing and wind events in shallow waters may result in benthic microalga resuspension, effectively mixing the two communities. While several estuaries have been well-studied, there remain many systems in which little or nothing is known about the microalgae. Species composition and community dynamics vary between estuaries, and are dependent on salinity distribution, turbidity, light penetration, nutrient loading, and mixing characteristics. Phytoplankton biomass rarely exceeds 50 mg chl a m23, but extensive phytoplankton blooms do occur in some systems, particularly in North Carolina and Florida (Mallin 1994; National Oceanic and Atmospheric Administration 1996). Although nitrogen is the macro-nutrient most commonly limiting to phytoplankton population growth in southeastern estuaries, limitation by silicate or phosphorus has also been reported, particularly in the more southern regions (south of Cape Romain; National Oceanic and Atmospheric Administration 1996). Light can also be an important limiting factor, and may be a more influential regulatory factor than nutrients in shallow and very turbid South Carolina and Georgia salt marsh estuaries (Pomeroy et al. 1981; Vernberg 1993; Lewitus et al. 2000). Microzooplankton grazing and iron have been also been suggested as limiting factors in certain South Carolina salt marsh estuaries (Kawaguchi et al. 1997; Lewitus et al. 1998). In North Carolina, phytoplankton production and abundance typically exhibit peaks in the March–May and July–September periods (Carpenter 1971; Thayer 1971; Mallin 1994). However, periodic winter blooms of estuarine dinoflagellates and cryptomonads, linked to nitrate loading pulses, occur in some estuaries in North Carolina (Mallin 1994; Mallin and Paerl 1994; Pinckney et al. 1998). Diatoms generally dominate the spring bloom phytoplankton communities in North Carolina (Dardeau et al. 1992; Mallin 1994; Tester et al. 1995), but dinoflagellates and cryptophytes can also be prevalent during all seasons and can dominate during the summer (Mallin 1994). Although Southeast Atlantic U.S. Estuaries 805 TABLE 3. Phytoplankton primary production in southeastern Atlantic estuaries. Estuary Pamlico, North Carolina Neuse, North Carolina Beaufort, North Carolina Murrells Inlet, South Carolina North Inlet, South Carolina Sapelo Island, Georgia Indian River, Florida Biscayne Bay, Florida Primary Production (g C m22 yr21) Source 288–500 280–370 52–68 76 100–355 190–375 328–441 13–46 Mallin (1994) Mallin (1994) Thayer et al. (1975) Lewitus unpublished data Pinckney and Zingmark (1993); Lewitus unpublished data Pomeroy et al. (1981) Jensen and Gibson (1986) Roman et al. (1983) phytoplankton dynamics in South Carolina and Georgia estuaries have been evaluated for only a few systems (Table 3), some distinctions from North Carolina estuaries are suggested below. However, because several North Carolina estuaries are moderately-to-highly eutrophic as a result of anthropogenic practices (Bricker et al. 1999), it is difficult to sort differences due to anthropogenic influences from those due to geography. Nutrient loading may be a much more influential driving force for regulating phytoplankton dynamics in North Carolina, especially in poorly flushed areas, than in the South Carolina and Georgia systems that are weakly-to-moderately eutrophic and typically well-flushed. In this respect, the prevalence of high dinoflagellate and cryptophyte populations intermittently throughout the year in North Carolina estuaries may result largely from the influence of weather-driven loading of anthropogenically-derived nutrients. In those South Carolina and Georgia estuaries studied, diatoms were the predominant contributors to phytoplankton biomass during much of the year (Marshall 1985; Verity et al. 1993; Lewitus et al. 1998). However, during the summer bloom, the relative contribution of phytoflagellates approached that of diatoms, and, in terms of abundance, the phototrophic nanoflagellates (several groups such as chrysophytes, prasinophytes, prymnesiophytes, cryptophytes, and dinoflagellates) and picoplankton (primarily coccoid cyanobacteria such as Synechococcus spp.) can exceed that of diatoms. Lewitus et al. (1998) found that microplanktonic diatom abundance in North Inlet estuary decreased from spring to summer, while phototrophic nanoplankton increased, suggesting that the bloom formed as a result of an increasing standing stock of the latter group. The bloom was characterized by a ‘‘microbial loop’’ structure; similar to summer bloom communities in more northern temperate estuaries such as Chesapeake Bay. Phytoplankton population growth was not limited by nitrogen, but rather controlled by microzooplankton grazing. In comparison to more northern tem- perate estuaries, the relative predominance of summer phytoflagellate-dominated bloom communities, with microbial loop dynamics and microzooplanktonic grazing control, may be a result of the shorter spring/prolonged summer characterizing the lower latitudes, which favors the regeneration of N-nutrients such as NH4 and DON, bacterial production, the growth of flagellates over diatoms, and grazing activity of microzooplankton over macrozooplankton (Glibert et al. 1982; Sherr et al. 1986, 1992; Keller et al. 1999; Lomas and Glibert 1999). Benthic microalgae are found on tidal flats, the surface of salt marshes, and subtidal areas. Their biomass may be as high as 250 mg chl a m22 in the upper 5 cm of sediment, but typically ranges from 10 to 100 mg chl a m22 depending on sediment type and depth of overlying water (Pinckney and Zingmark 1993b; Sigmon and Cahoon 1997; Cahoon et al. 1999). A study of five salt marsh habitats (Pinckney and Zingmark 1993b) found the highest benthic algal biomass in sediments within Spartina habitats and on intertidal mudflats, and lowest biomass in shallow subtidal areas. In a broad scale study that included the subtidal and intertidal areas of eight North Carolina estuaries, Cahoon et al. (1999) found a negative relationship between the proportion of fine sediments and benthic microalgal biomass. Annual benthic microalgae production ranges from about 50 to over 200 g C m22 (Pomeroy 1959; Pinckney and Zingmark 1993a). In North Inlet, South Carolina, combined microalgal productivity can be as high as 50% of the total macrophyte primary production (Pinckney and Zingmark 1993a). Additional primary production can be attributed to epiphytic microalgae. The production of epiphytic microalgae in southeastern estuaries is highly variable and species-specific (Coleman and Burkholder 1994), but can range from 20% to 200% of that of host seagrasses or marsh macrophytes (Penhale 1977; Mallin et al. 1992). 806 R. Dame et al. TABLE 4. Distribution of salt marshes along the southeastern Atlantic coast. Area (ha) State North Carolina South Carolina Georgia Florida Spartina Juncus Total 42,923 112,766 121,335 10,536 40,712 36,882 30,337 28,304 83,635 149,648 151,672 38,840 HARMFUL ALGAL BLOOMS Increasing anthropogenic nutrient inputs from a rapidly growing human population in the coastal zone, industrial and municipal effluents, changing land-use practices, and atmospheric nitrogen deposition are rapidly exceeding the processing ability of impacted systems (Paerl 1997). Symptoms of eutrophication (nuisance and toxic algal blooms, hypoxia/anoxia, fish and shellfish mortality) have been documented in nearly half of major southeastern estuaries (Bricker et al. 1999). Harmful algal blooms such as red tides or brown tides occur infrequently in most southeastern Atlantic coastal systems. Outbreaks of red tides caused by Gymnodinium breve are more common along the southern portion of the area; however, in 1987 one was carried by the Gulf Stream as far north as Onslow Bay in North Carolina (Tester et al. 1991). Cyanobacterial (blue-green algal) and dinoflagellate blooms have resulted in significant ecological and economic damage in some rivers and estuaries of North Carolina and Florida. In the late 1980s, a group of toxic dinoflagellate species were found in several North Carolina estuaries (Burkholder et al. 1992). One recognized species in this group, Pfiesteria piscicida, has been identified as the causative agent of approximately 50% of the fish kills in the Pamlico, Neuse, and New River estuaries of North Carolina in the past decade (Burkholder and Glasgow 1997; Burkholder et al. 1995; Burkholder 1998). A second, less virulent toxic Pfiesteria species has also been cultured, identified, and tested for toxicity from waters of this region (Burkholder and Glasgow 1997). Both species have also been found in sub-estuaries of the Chesapeake Bay (Lewitus et al. 1995). Reports of harmful algal blooms in South Carolina and Georgia estuaries are rare. To our knowledge, Pomeroy et al.’s (1956, 1972) report of a dinoflagellate bloom (Kryptoperidinium sp.) in the Sapelo Island salt marsh estuary is the only potential harmful algal bloom documented in Georgia estuarine waters. The first reported red tide localized to a South Carolina estuary occurred in Spring 1998, when a new species of dinoflagellate (Scrippsiella carolinium, Morton personal communication) formed a bloom in Bulls Bay, McClellanville (Lew- Source Critcher 1967 Alexander et al. 1986 Alexander et al. 1986; Eleuterius 1976 Eleuterius 1976 itus et al. in press). This same species formed red tides in several sites in spring 1999 ranging from North Inlet to Hilton Head Island estuaries over 100 miles apart. Although the environmental factors promoting harmful algal blooms are not wellunderstood, and events are unpredictable and vary with species, the relative lack of harmful algal blooms in the estuaries of South Carolina and Georgia may relate to the high tidal flushing, short water residence time, and low anthropogenic nutrient loading that characterize these systems. The recent, recurrent (1998–1999), and widespread appearance of the Scrippsiella red tide in South Carolina is worrisome, and it is unknown whether its origin was related to natural events or anthropogenic influence. In contrast, high nutrient loading and long water residence times characterize the sites where Pfiesteria-induced fish kills tend to occur (Burkholder et al. 1995; Burkholder and Glasgow 1997). Tidal flushing and short water residence times in many southeastern systems may minimize the occurrence of both toxic and nuisance phytoplankton blooms. In order to prevent or control harmful algal blooms in the region, the development of ecologically sound and economically feasible watershed-based nutrient management strategies must be addressed in the coming years. MACROALGAE The southeastern Atlantic coast of the U.S. has been characterized as inhospitable to macroalgae (seaweeds) and without its own flora (Humm 1969; Coutinho 1987). For example, in Georgia and South Carolina estuaries, macroalgae is primarily found in shallow creeks during the winter months when turbidity levels are low. The typical distribution of macroalgae in estuaries is that of marine species colonizing most of the length of the estuary with freshwater species predominating only near the head (Wilkinson 1980). In North Inlet, South Carolina, the highest numbers of macroalgal species were found in winter, with peak reproductive activity occurring during the spring (Coutinho 1987). The area averaged annual net primary production of macroalgae in North Inlet is 197 g C m22 (Coutinho 1987). The magnitude of macroal- Southeast Atlantic U.S. Estuaries 807 TABLE 5. Vascular plant and macroalgal primary production for several estuaries along the southeastern Atlantic coast. Estuary Beaufort, North Carolina North Inlet, South Carolina Form Sapelo Island, Georgia seagrass marsh grass macroalgal marsh grass Indian River, Florida Biscayne Bay, Florida seagrass seagrass gae production is usually low relative to vascular plant production in the area (Table 4). VASCULAR PLANTS Vascular plants common to estuaries along the southeastern Atlantic coast include salt marshes, sea grasses, and mangroves. The intertidal salt marsh grass Spartina alterniflora is the dominant species in most estuaries from North Carolina through Georgia (Table 4). In north Florida, Juncus marshes tend to dominate at higher intertidal elevations. Salt marshes reach their greatest extent in Georgia and South Carolina. South of Daytona Beach, the black mangrove, Avicennia germinans, in the intertidal zone, gradually replaces S. alterniflora. Spartina alterniflora is the most common salt marsh plant with distinctive height forms depending on location in the marsh. The dominant vegetation shifts as salinity changes from salt to freshwater (Odum et al. 1984). Spartina alterniflora, S. cynosuroides, and Juncus roemaerianus are important in brackish water, and Zizaniopsis milacea, Zizanaia aquatica, Pontederia cordat, Scirpus validus, Eleocharis albida, Peltandra virginicus, and Typha dominogensis occur in tidal freshwater marshes (Gallagher and Reimold 1973; Schubauer and Hopkinson 1984). Hefner et al. (1994) recently reported that there was almost no salt marsh wetland loss along the southeastern Atlantic coast in the 1970s to 1980s. Annual net primary production of S. alterniflora (above 1 belowground) ranges from 0.1 to over 2.5 kg C m22 in short form stands and 0.8 to 2.1 kg C m22 for stands of the tall form (Dame 1989; Montague and Wiegert 1990; Dai and Wiegert 1996). Highest values are in South Carolina and Georgia with low values in North Carolina and Florida (Table 5). In south Florida, Rhizophora mangle, red mangrove, is the most typical mangrove plant, particularly in areas heavily influenced by seawater. The structure of mangrove swamps is usually attributed to topography, substrate, tidal action, and freshwater hydrology (see Lugo and Snedaker 1974). Moving up riverine systems into lower salinities and higher intertidal elevations, other vascular Primary Production (g C m22 yr21) 134–700 800–2,100 197 815–2,000 749–1,421 159 520–2,000 Source Thayer et al. 1975; Penhale 1977 Dame 1989; Coutinho 1987 Pomery et al. 1981; Dai and Wiegert 1996 Jensen and Gibson 1986 Roman et al. 1983 plant species besides R. mangle can be found. Avicennia, black mangrove, and Laguncularia, white mangrove, are common to this group. Mangrove annual net primary production ranges from 0.4 to 4.4 kg C m22 with an average of 1 kg C m22 (Mitsch and Gosselink 1993; Odum and McIvor 1990). Seagrasses are found in the northernmost estuaries of North Carolina and in Florida. In many areas, there has been a drastic decline in seagrass abundance that has been attributed to declining water quality. These subtidal vascular plants inhabit soft sediments and appear to be light limited (Day et al. 1989) which may be a primary cause for their absence in South Carolina and Georgia estuaries. In North Carolina, the dominant form is eelgrass, Zostera marina, and in Florida turtlegrass, Thalassia testudinum, dominates. Seagrass annual net primary production (Table 5) in North Carolina ranges from less than 0.1 to 0.7 kg C m22 and in Florida from 0.1 to 2.5 kg C m22 (Thayer et al. 1975; Penhale 1977; Zieman and Wetzel 1980; Zieman 1982). TOTAL PRIMARY PRODUCTION There have been estimates of the various components of total primary production in only a few locations within the southeastern Atlantic coastal region (Table 6). In the estuarine area near Beaufort, North Carolina, Thayer et al. (1975) estimated a total annual primary production of 0.2 kg C m22 distributed among eelgrass (64%), phytoplankton (28%), and salt marsh grass (8%). At North Inlet, South Carolina, estimated total annual primary production including belowground rootrhizome production is 1.0 to 1.6 kg C m22 (Dame 1989; Pinckney and Zingmark 1993a). Using minimal values and adjusting for the total system, annual production is partitioned as S. alterniflora (46%), benthic microalgae (29%), macroalgae (13%), and phytoplankton (12%). In the Duplin River system near Sapelo Island, Georgia, total annual primary production was estimated to be 1.4 kg C m22 apportioned as S. alterniflora (84%), benthic microalgae (10%), and phytoplankton (6%). Pomeroy et al. (1981) suggests that on the bottom 808 R. Dame et al. TABLE 6. Relative primary production at five estuaries along the southeastern Atlantic coast. Data sources given in text. Relative Primary Production (%) Estuary Beaufort,North Carolina North Inlet, South Carolina Sapelo, Georgia Indian River, Florida Biscayne Bay, Florida Phytoplankton Benthic Microalgae 33.7 12.0 7.6 3.5 6.7 and in the water column, turbidity limits light penetration and, thus, photosynthesis. In Biscayne Bay, Florida, Roman et al. (1983) showed that phytoplankton (27 g C m22 yr21) accounted for less than 10% of total system production with the seagrass Thalassia and seaweeds accounting for the majority (0.6 kg C m22 y21). From these widely distributed sites, it appears that macrophytes are by far the dominant producer of fixed carbon in southeastern estuaries. Phytoplankton production is relatively low except at Beaufort, North Carolina where it accounts for over one-fourth of the total production of the system. Some eutrophic systems such as the Neuse River differ from the above systems; the main source of fixed carbon is phytoplankton because seagrasses are rare or absent and salt marshes are confined to river fringes in lower reaches of the estuary. The importance of vascular plant primary production in many southeastern estuarine ecosystems supports Peters and Schaaf’s (1991) empirical modeling exercise that suggests that there is insufficient microalgae production to support the current catch of finfish and shellfish along the Atlantic coast of the U.S. Opposing views exist that argue the significance of algae (particularly edaphic microalgae) to carbon trophic transfer in southeastern estuarine food webs. The Peters and Schaaf (1991) model probably underestimated the relative contribution of algae to fish production by using a 20% trophic transfer efficiency, which does not account for the need for vascular plant detritus, and is high relative to the prevalent 10% efficiency found by Pauly and Christensen (1995) in their worldwide survey of primary productivity needs for fishery sustenance, and failing to account for the production of edaphic microalgae in salt marshes and seagrass beds (Mallin et al. 1992). Also, stable isotope analyses from the Sapelo Island salt marsh (Haines 1976; Peterson and Howarth 1987; Sullivan and Moncreiff 1990) indicated that algae accounted for 50% of the transfer of fixed carbon to fauna, despite their much lower absolute primary production compared to vascular plants; i.e. suggesting that the efficiency of trophic trans- 29.0 14.4 14.6 Macroalgae 13.0 Vascular Prorated Total (g C m22 yr21) 66.3 46.0 78.0 81.9 93.3 202 1,600 1,445 441 686 fer is higher in algae than vascular plants in this system. Consumers With their high primary production, estuaries of the southeastern Atlantic coast also have abundant and often commercially important consumers. Because macrophytic production dominates the estuaries of the region, both detrital and grazing food paths are well represented. Frequently, many macro-consumers are feeding on organisms from both of these pathways. Furthermore, many consumers are predominantly found in either bottom sediment or water column habitats, but mechanisms coupling these subsystems abound. MICROCONSUMERS The decomposer food web in coastal waters is dominated by microorganisms that are either using DOM in the water column or particulate organic matter (POM). The community of attached microbial organisms and POM is usually referred to as detritus. Organic carbon enters estuaries from other environments and from macrophytes, epiphytes, benthic microalgae, macroalgae, and phytoplankton. In North Inlet, DOC concentrations range from 1.0 to 13.0 g C m23 and particulate organic carbon (POC) concentrations vary greatly about seasonal mean concentrations of 1.2 to 1.5 g C m23 (Dame et al. 1986). In the Duplin River near Sapelo Island, DOC varies over the year from 2 to about 11 g C m23 and POC range from 3 to 11 g C m23 over the same period (Chalmers et al. 1985). Both systems trap sufficient particulate carbon (organic and inorganic) to maintain elevation as sea level rises and export carbon to the adjacent ocean. The source of the majority of dissolved and particulate carbon in southeastern Atlantic coast estuaries is usually attributed to the decomposition of organic materials. Odum and de la Cruz (1967) gave the original description of the aerobic decomposition of Spartina alterniflora for conditions at Sapelo Island. After the initial leaching stage of decomposition, during which a large pulse of DOM is released from post senescent tissues (Valiela et Southeast Atlantic U.S. Estuaries 809 TABLE 7. Comparison of means zooplankton abundance and biomass for estuaries along the southeastern Atlantic coast. Estuary Beaufort area, North Carolina Neuse River, North Carolina North Inlet, South Carolina Biscayne Bay, Florida Plankton Net Mesh Size (mm) 156 76 76 60 156 153 64 and 200 239 Density (No. m23) Sample Period 1970 1971 30 mo 20 mo 1989 22 mo 20 mo 6 mo (summer) 1978 14 mo al. 1985), fibrous tissues are initially colonized by fungi and bacteria that structurally weaken the tissues so that macroconsumers can process them into smaller particles (Newell 1996). As the particles get smaller the surface area increases and the microbes increase their colonization. The original vascular plant material is gradually converted into microbial biomass and CO2 as a byproduct. Various detrital feeders then ingest these small particles, the microbes are removed, and the POM repackaged as fecal pellets that are egested, and then recolonized by microbes with the particles again ingested by consumers. A comparison of vascular plant decomposition shows that the seagrasses decompose the fastest, followed by red mangroves, and salt marsh grasses the slowest (Odum and de la Cruz 1967; Heald 1969; Zieman 1975). In addition to the decomposition of organic material aerobically, much vascular plant material is also buried in estuarine sediments where it decomposes anaerobically and plays a role in a number of bacterially mediated biogeochemical cycles. In the water column, the microbial food web has been identified as a major pathway for processing of DOM (Azam et al. 1983). In this web, DOM produced by various sources, both living and non-living, is taken up by bacteria that are then consumed by protozoans and finally the protozoans are eaten by macroconsumers (LeGall et al. 1997). DOM from producers may also be converted, via microbial mediation, to organic aggregates that are in turn consumed by metazoa (Alber and Valiela 1995). The role of microzooplankton (e.g., protists such as flagellates and ciliates) in processing bacterial or primary production is unknown for many estuaries in the southeast, and in fact most information comes from two salt marshes; Sapelo Island, Georgia, and North Inlet, South Carolina. In both systems, estimates of abundances and grazing rates of microzooplankton were at the high end of those reported in the literature (Sherr et al. 1986, 4,000 8,400 21,000 34,530 31,224 137,150 9,257 21,555 47,000 Biomass (mg dry wt m23) 14.0 21.0 47.8 15.3 17.2 38.7 16.1 — 25 53.4 Source Thayer et al. (1974) Thayer et al. (1974) Fulton (1984) Mallin (1991) Mallin (1991) Mallin and Paerl (1994) Lonsdale and Coull (1977) Houser and Allen (1996) Roman et al. (1983) Woodmansee (1958) 1991; Capriulo 1990; Zeitzschel 1990; Sanders et al. 1992; Wetz et al. 1999). Sherr et al. (1986, 1989, 1991) estimated that, depending on season and proximity to the land-margin, small (, 20 mm) aloricate ciliates or heterotrophic flagellates were the major bacterivore. Lewitus et al. (1998) demonstrated the importance of microzooplankton grazing in limiting phytoplankton population growth during the summer bloom in North Inlet. Heterotrophic flagellate abundance peaked at 6,000 to 9,000 cell ml21 during the summer in several North Inlet tidal creeks (Wetz et al. 1999). These numbers are exceptionally high relative to literature values, consistent with the high bacterial abundance typically measured during the summer in North Inlet (up to 108 cell ml21; Lewitus et al. 2000). Research is need to determine whether the relatively high heterotrophic flagellate, ciliate, and bacterial abundances, and the predominance of microzooplankton grazing in controlling bacterial and phytoplankton population growth, are characteristic of other southeast estuaries (and, thus, may reflect the generally higher temperatures and prolonged summer compared to more northern temperate estuaries). MACROCONSUMERS Macroconsumers of southeastern Atlantic coast estuaries are abundant, diverse, and often commercially important. In the water column, the macroconsumers are mainly zooplankton and nekton. Many of these animals are meroplanktonic with larval stages that live in the water column and adult stages that are nektonic or benthic (Dame and Allen 1996). There are only a few estimates of annual zooplankton density and biomass in southeastern estuaries; most of these being from North and South Carolina (Table 7). These data represent contrasting estuarine geophysical types, with Beaufort, North Carolina estuaries consisting of several interconnected high-salinity sounds, the Neuse a mesohaline riverine system, North Inlet a high-sa- 810 R. Dame et al. linity marsh-estuarine system, and Biscayne Bay a subtropical open body of water. Recorded zooplankton abundance was highly dependent upon net mesh size used in collections, with use of smaller (60–76 mm) mesh sizes yielding much greater abundance and biomass (Table 7). Season was also important in these systems with summers yielding the highest abundances. Fulton (1984), Mallin (1991), and Mallin and Paerl (1994) found a positive correlation between water temperature and zooplankton abundance and biomass. At the southern end of the range, Roman et al. (1983) attributed the higher zooplankton concentrations in Biscayne Bay to the availability of large amounts of macroalgal detritus. It should also be noted that these subtropical estuarine waters are considerably less turbid than those of the more temperate systems. Mallin and Paerl (1994) estimated that zooplankton grazed between 38% and 45% of daily phytoplankton production on an annual basis in the Neuse River Estuary in North Carolina. Holoplanktonic consumers (e.g., copepods) are primary sources of food for fish (Weinstein 1979; Bozeman and Dean 1980) and zooplanktivorous fishes (Allen et al. 1995) that arrive in the estuarine nursery ground in the spring. Predation peaks in the spring and summer when the transfer of phytoplankton carbon to zooplankton is highest, and most fishes depart in the autumn when phytoplankton productivity and zooplankton abundance have decreased (Mallin and Paerl 1994). Benthos composition and abundance generally reflects the latitude, type, and geomorphology of estuaries within the region. Sediments, currents, salinity, temperature, and many other abiotic and biotic factors influence the benthos over a wide range of spatial and temporal scales. Infauna and epifauna associated with salt marshes are similar from North Carolina to Florida. Species composition and abundance varies within each estuary as a function of elevation within the tidal range, bottom type, and distance from the ocean. Low energy environments (mud-silt) typically support higher diversities of meiofauna than higher energy sandy habitats (Coull 1985). Although macrobenthos biomass often surpasses that of meiofauna, the smaller animals are more diverse and have much higher turnover rates (Coull and Bell 1979). Microcrustaceans and oligochaetes are the primary source of food for shrimps, fishes, and other epibenthic species that use the estuarine nursery. Polychaetes dominate the macrobenthos in mud bottoms, whereas amphipods and small bivalves are most abundant on sand bottoms, and echinoderms may dominate carbonate sediments (Dardeau et al. 1992). Seasonal variations in nu- merical abundance and species richness may be large with maxima usually occurring between fall and spring (Service and Feller 1992). Hard bottoms are not abundant within the region, but where they do occur, they are often very important. Benthic filter feeding bivalves often comprise the most obvious consumer community forming dense beds and, in the case of oysters, reefs with complex structural attributes. Intertidal oyster reefs are common to bar-built and lagoonal systems throughout the region. In North Inlet they have been shown to be capable of removing more phytoplankton from the water column than is produced by the estuary, resulting in a regular total import of phytoplankton from the adjacent ocean (Dame et al. 1986, 1989). At North Inlet, Dame et al. (1989) calculated that zooplankton grazing was several orders of magnitude below oyster grazing on phytoplankton. Because of their high rates of water pumping and filtration, oyster reefs have also been shown to play a major role in the cycling of nutrients in these systems (Dame 1996). Benthic diversity and productivity are especially high in vegetated subtidal areas. Seagrass beds in North Carolina and south Florida are habitats for hundreds of species of benthic, epiphytic, and mobile invertebrates (Dardeau et al. 1992). Dominated by polychaetes and amphipods, the density and diversity of benthos in these habitats increases towards the south (Virnstein et al. 1984). Mangrove habitat supports a distinct, speciose, and productive benthos and epifauna in the southern portion of the region (Odum and Heald 1972). Mobile macroconsumers or nekton are also prominent components of southeastern Atlantic estuaries. Whereas, many of these animals are permanent (year round) residents, warm season transients (mostly young of the year fish and shrimps) often account for most of the mobile annual consumers in these estuaries. Estuarine nekton use the shallow tidal creeks, marshes, mangrove swamps, and vascular plant grass beds as nursery grounds and habitat (Hettler 1989; Gilmore 1995; McIvor and Rozas 1996). Resident and transient nekton can potentially move production from these shallow habitats into the subtidal estuary and coastal ocean (Kneib 1997). Nelson et al. (1991) surveyed 20 mostly riverine estuaries on the southeastern Atlantic coast for the distribution and abundance of fishes and shellfish. Sufficient information about commercial value, recreational value, indicators of environmental stress, and ecological value for about 40 species were available to develop a general analysis. Penaeid shrimps and blue crabs dominated the invertebrate component of the nekton, and both groups have life cycles that strongly couple the estuary to the coastal ocean. Blue crabs Southeast Atlantic U.S. Estuaries 811 Fig. 7. A comparison of fisheries landings in North Carolina, South Carolina, Georgia, and Eastern Florida for 1988–1998. Fig. 9. Total commercial fish and shellfish landings in Georgia from 1988–1998. were abundant or highly abundant in all systems across the region, while the abundance of shrimp varied with different species dominating across the region. The most abundant finfish (the bay anchovy, spot and striped mullet) occur across a wide range of salinities and dominate most southeastern estuaries. In a 16-year continuing study, Allen (personal communication) found that young of the year of dominant nekton species in North Inlet varied considerably in abundance and biomass from year-to-year, but that the total production of nekton seemed to be much less variable. A review of commercial landings of finfish and shellfish (shrimp, crabs, and bivalves) data from 1988 to 1998 for each state in the region showed that total catch per year was stable with North Carolina’s loadings being greater than those of the other three states combined (Fig. 7). Finfish dominate the catches in North Carolina and Florida (Figs. 8 and 9), while the greater proportion of catch in South Carolina and Georgia is shellfish (Figs. 10 and 11). In North Carolina, South Carolina, and Georgia, estuarine related finfish and shellfish species dominated the catch, but in Florida, catches were slightly dominated by marine species (Fig. 12). The commercial landings data support the idea that the South Carolina and Georgia fisheries are very similar both in terms of total catch and proportion of shellfish and estuarine species, that North Carolina’s fisheries are dominated by finfish, have greater annual yields compared to South Carolina and Georgia, but are also primarily based on estuarine species, and that Florida’s fisheries are more equally divided between shellfish and finfish, but estuarine species account for less than half the catch. This characterization of the southeastern estuaries by states corresponds to the geomorphological distribution of sounds dominating in North Carolina; barrier islands with bar-built estuaries most common in South Carolina and Georgia and marine factors dictating in Florida. These data indicate a relationship between dif- Fig. 8. Total commercial fish and shellfish landings in North Carolina from 1988–1998. Fig. 10. Total commercial fish and shellfish landings in eastern Florida from 1988–1998. 812 R. Dame et al. Fig. 11. Total commercial fish and shellfish landings in South Carolina from 1988–1998. fering levels of primary production and fisheries harvest across the region, which is consistent with ecological theory regarding food chain structure and productivity (Pauley and Christensen 1995). At the top of southeastern estuarine food webs are a number of highly mobile predators including raptorial birds, sharks and rays, turtles, alligators and crocodiles, as well as marine mammals, the most dominant of which is the bottle nosed dolphin (Hoese 1971). Without any significant predators, these animals with highly developed sensory systems and behaviors are well adapted to feeding on larger fish, shrimp, and turtles in the highly turbid shallow estuarine waters of the region. Anthropogenic Effects Human population density has steadily increased along the southeastern Atlantic coast since Europeans first colonized it centuries ago. Over the 30-yr period between 1980 and 2010, human population density in coastal counties of the region is expected to continue to rise (Fig. 13) with the average growth rate by state expected to range between 15% and 45% with Florida leading the way (Culliton et al. 1990). In Florida, coastal counties occupy 57% of the land, but contain 78% of the population (Montague 1997; Montague and Odum 1997). With a human population growth rate of over 600 individuals per day, Florida is already exhibiting environmental stress in many areas. At this rate of human population growth, it is projected that Florida will lose the ability to sustain its estuarine environments within the next 20 years (Montague and Odum 1997). In contrast to Florida, Georgia and South Carolina have some of the smallest coastal human populations in the country, but their growth rates are still high (10% to 20% per decade). In the Carolinas and Georgia, there Fig. 12. Proportion of total fisheries landings comprised of finfish and shellfish species associated with estuaries along the southeastern Atlantic coast for 1988–1998. is also a well-defined seasonal change in population due to recreational tourism. The net effect of millions of tourist visiting this area annually is the doubling of the average daily population. Development of infrastructure has had significant environmental impact. Along the northeast coast of South Carolina there are over 110 golf courses that are essentially intensely maintained grasslands developed in response to human recreational demands. In addition to problems associated with routine management, there is mounting concern that golf course ponds will not be able to contain their contents during a significant hurricane induced storm surge. Fig. 13. Past and projected human population numbers in coastal counties of the southeastern Atlantic coast. Data sources: North Carolina Office of State Planning, State Demographics; South Carolina Budget and Control Board, Office of Research Statistics; Georgia GIS Data Clearinghouse and State Data and Research Center; and Florida Office of Economic and Demographic Research, The Florida Legislature. 813 Southeast Atlantic U.S. Estuaries TABLE 8. Southeast Atlantic estuarine eutrophication assessment. L 5 LOW, ML 5 Moderate Low, M 5 Moderate, MH 5 Moderate High, H 5 High, IH 5 Improved High, IL 5 Improved Low, NC 5 No Change, WL 5 Worse Low, WH 5 Worse High (Coastal Assessment and Data Synthesis System 1999; National Oceanic and Atmospheric Administration 1999). Eutrophication Symptoms Estuary L ML Albemarle Sound Pamlico Sound Pamlico/Pungo Rivers Neuse River Bogue Sound New River Cape Fear River Winyah Bay/North Inlet North/South Santee Rivers Charleston Harbor/Wando/Cooper/Ashley Rivers Stono/North Edisto Rivers St. Helena Sound/S. Edisto/Coosaw Rivers Broad River Savannah River Ossabaw Sound/Ogeechee River St. Catherines/Sapelo Sounds Altamaha River St. Andrew/St. Simons Sounds/Satilla River St. Marys River/Cumberland Sound St. Johns River Indian River Biscayne Bay X X South Atlantic Coast 11 1 M MH Anthropogenic Influences H L ML M MH H Prognosis (2020) IH IL X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X X Estuarine water quality is often used as an indicator of environmental stress in coastal areas with nutrient concentrations being the most common measure (National Oceanic and Atmospheric Administration 1996). The sources of additional nutrients to estuaries are point (e.g., wastewater treatment, industry, etc.) and non-point (e.g., agriculture, golf courses, lawns, etc.) discharges. These inputs have both direct effects (i.e., algal blooms, increased turbidity, decreased oxygen concentrations, etc.) and indirect effects (i.e., decreases in commercial fisheries, impacts on recreation and public health risks) (National Oceanic and Atmospheric Administration 1996). A recently published National Oceanic and Atmospheric Administration (1996) report focused on existing information on nutrient loading or eutrophication in 21 south Atlantic estuaries (Table 8). Medium concentrations (. 5 mg l21) of chl a are observed periodically in 20 estuaries and 11 of these systems had high or hypereutrophic (. 20 mg l21) concentrations episodically. Medium or greater (. 0.1 mg l21) concentrations of nitrogen are found in 19 estuaries. Phosphorus concentrations of medium or greater (. 0.01 mg l21) are observed in 18 estuaries. During the summer months small percentages of 13 estuaries are hypoxic and 11 systems exhibit anoxic occurrences. The trends data were sparse, but the Neuse River 3 X X X X X X X X 5 2 5 WH X X X X X WL X X X NC 5 8 2 1 0 3 8 4 7 appeared to have a number of increasing and decreasing trends depending on location within the estuary. In contrast, data from a number of South Carolina estuaries indicated a decrease in nitrogen and phosphorus loading. As noted earlier, water flow and watershed physical characteristics play a major role in estuarine dynamics. Biggs et al. (1989) developed a classification scheme designed to evaluate the potential ability of an estuary to flush pollutants. From this scheme, the Santee River in South Carolina is most susceptible to population, heavy industry, and agricultural activities. Fortunately, the Santee has no industry or large cities, but is mainly an agricultural watershed. The St. Johns River is projected to be most susceptible to population and agricultural activities. Charleston Harbor would be most susceptible to heavy industry, while Winyah Bay, Albemarle Sound, and the Neuse River would be most susceptible to agricultural activities. Unfortunately for the latter 5 estuaries, the scheme predicts these system’s actual situation. Conclusions This review of the characteristics of southeastern Atlantic estuaries has taken an ecosystem approach to include as many components and cross discipline interactions as possible. Three types of estuaries play major roles in the region. The marshes 814 R. Dame et al. in all three system types (bar-built, coastal plain, and piedmont) have high primary production and provide extensive nursery grounds for secondary consumers. Both coastal plain and piedmont systems flush extensive, highly productive freshwater wetlands. Piedmont estuaries, with large water flows, connect the coast to large interior watersheds. These highly productive estuarine systems can be both directly and indirectly influenced by management decisions both locally and as far away as the foothills of the Appalachian mountains. The discharges of the southeastern riverine systems carry large quantities of dissolved and particulate materials to the coastal ocean. Lagoonal and bar-built systems that are tidally dominated may export large amounts of inorganic and organic materials. Water fluxes in all three estuarine types appear to play a major role in the accumulation or lack of accumulation of anthropogenic materials, nuisance algae blooms, and marine diseases. Vascular plant production dominates estuaries of the region and is decomposed through extensive foodwebs in both the water column and the sediment. This high primary production combined with extensive vascular plant habitat supports large populations of commercially and recreationally important secondary consumers. The estuaries of the southeastern Atlantic coast can be divided into three general areas. The North Carolina coast is dominated by extensive and poorly flushed sounds. These estuaries are characterized by modest primary production and they support the largest estuarine fishery in the southeastern Atlantic region. Although human population density is low and growth is moderate, high nutrient loading results from poor land use for industrial animal production. This area harbors several of the most polluted estuaries in the country. The central region that makes up most of the South Atlantic Bight is dominated by the estuaries of South Carolina and Georgia. Well-flushed barbuilt and riverine estuaries are common. Primary production is very high and dominated by emergent salt marshes. Commercial fishery landings are relatively low, but shellfish dominate catches and estuarine species comprise more than 75% of the catch. Human population density and growth are low to moderate and nutrient loading reflects this land use pattern. The Florida coast, particularly south of Jacksonville, is dominated by moderate to poorly flushed bar-built estuaries with little freshwater input. A variety of plant types support a modest level of primary production. Fisheries catches are moderate, and equally divided between shellfish and finfish, but estuarine species are much less important than marine species in the landings. Human population density and growth rate are high and probably cause high nutrient loading. With continued high population growth, urbanization, and habitat destruction, Florida presents the scenario of potential environmental collapse. One of the points to emerge from this synthesis is that, although a few representative bar-built systems are very well studied (e.g., North Inlet, South Carolina and Duplin River, Georgia), very little is known about most of the estuaries in the southeast Atlantic region. In contrast to other areas of the country, this region has received relatively little attention and the data do not exist for evaluating historic trends for many parameters. As reflected in several of the federal government reports that are the results of scientific panels, the future utilization of these estuarine systems and their essential services will depend on the development of improved management strategies based on enhanced data quality. These approaches must be increased comprehensive long-term environmental monitoring linked to integrated regional planning in order to maintain the viability and usability of southeastern Atlantic estuarine and coastal systems. In particular the magnitude and sources of freshwater inputs, the nature of material processing by the different types of estuaries and how coastal fisheries are coupled to these estuaries clearly need comprehensive study. Most importantly there is an evident need for large or regional scale approaches focused on the estuarine coupling of the landscape to the sea. ACKNOWLEDGMENTS The authors wish to thank the many state, federal, and private resources that have supported their research on southeastern estuaries in the past and present. This work was heavily supported by National Science Foundation awards DEB-95095 to Coastal Carolina University and the Georgia Rivers Land Margin Ecosystem Research DEB-94120898 to the University of Georgia. This is publication number 1252 of the Belle W. Baruch Institute for Marine Biology and Coastal Research. This work was presented at the Estuarine Federation Meeting in Providence, Rhode Island, October 1997. LITERATURE CITED ALBER, M. 2000. Settable and non-settable suspended sediments in the Ogeechee River Estuary, Georgia USA. Estuarine, Coastal, and Shelf Science 50:805–816. ALBER, M. AND J. E. SHELDON. 1999. Use of a date-specific method to examine variability in the flushing times of Georgia estuaries. Estuarine, Coastal and Shelf Science 49:469–482. ALBER, M. AND I. VALIELA. 1995. Organic aggregates in detrital food webs: Incorporation by bay scallops, Argopecten irradians. Marine Ecology Progress Series 121:117–124. ALBERTS, J. J AND M. TAKACS. 1999. Importance of humic substances for carbon and nitrogen transport into southeastern United States estuaries. Organic Geochemistry 30:385–395. ALEXANDER, C. E., M. A. BRONTMAN, AND D. W. FIELD. 1986. An Inventory of Coastal Wetlands in the USA. National Oceanic Southeast Atlantic U.S. Estuaries and Atmospheric Administration National Ocean Service, Department of Commerce, Washington, D.C. ALLEN, D. M., W. S. JOHNSON, AND V. OGBURN-MATTHEWS. 1995. Trophic relationships and seasonal utilization of salt marshes by zooplanktivorous fishes. Environmental Biology of Fishes 42: 37–50. AZAM, F., T. FENCHEL, J. G. FIELD, J. S. GRAY, L. A. MEYER-REIL, AND F. THINGSTAD. 1983. The ecological role of water-column microbes in the sea. Marine Ecology Progress Series 10:257–263. BIGGS, R. B., T. B. DEMOSS, M. M. CARTER, AND E. L. BEASLEY. 1989. Susceptibility of U.S. estuaries to pollution. Reviews in Aquatic Sciences 1:189–207. BIGGS, R. B. AND B. A. HOWELL. 1984. The estuary as a sediment trap: Alternate approaches to estimating its filtering efficiency, p. 107–129. In V. S. Kennedy (ed.), The Estuary as a Filter. Academic Press, New York. BOZEMAN, E. L. AND J. M. DEAN. 1980. The abundance of estuarine larval and juvenile fish in a South Carolina intertidal creek. Estuaries 3:89–97. BRICKER, S. B., C. G. CLEMENT, D. E. PIRHALLA, S. P. ORLANDO, AND D. R. G. FARROW. 1999. National Estuarine Eutrophication Assessment: Effects of Nutrient Enrichment in the Nation’s Estuaries. National Oceanic and Atmospheric Administration, National Ocean Service, Special Projects Office and the National Centers for Coastal Ocean Science. Silver Spring, Maryland. BURKHOLDER, J. M. 1998. Implications of harmful marine microalgae and heterotrophic dinoflagellates in management of sustainable marine fisheries. Ecological Applications 8:S37-S62. BURKHOLDER, J. M. AND H. B. GLASGOW, JR. 1997. Pfiesteria piscicida and other Pfiesteria-like dinoflagellates: Behavior, impacts, and environmental controls. Limnology and Oceanography 42:1052–1075. BURKHOLDER, J. M., H. B. GLASGOW, JR., AND C. W. HOBBS. 1995. Distribution and environmental conditions for fish kills linked to a toxic ambush predator dinoflagellate. Marine Ecology Progress Series 124:43–61. BURKHOLDER, J. M., E. J. NOGA, C. H. HOBBS, H. B. GLASGOW, AND S. A. SMITH. 1992. New ‘phantom’ dinoflagellate is the causative agent of major estuarine fish kills. Nature 358:407– 410. CAHOON, L. B., J. E. NEARHOOF, AND C. L. TILTON. 1999. Sediment grain size effect on benthic microalgal biomass in shallow aquatic ecosystems. Estuaries 22:735–741. CAI, W. J., L. R. POMEROY, M. A. MORAN, AND Y. WANG. 1999. Oxygen and carbon dioxide mass balance for the estuarineintertidal marsh complex of five rivers in the southeastern U.S. Limnology and Oceanography 44:639–649. CAI, W. J., W. J. WIEBE, Y. WANG, AND J. E. SHELDON. in press. Intertidal marsh as a source of dissolved inorganic carbon and a sink of nitrate in the Satilla River-estuarine complex in the southeastern U.S. Limnology and Oceanography. CAPRIULO, G. M. 1990. Feeding-related ecology of marine protozoa, p. 186–259. In G. M. Capriulo (ed.), Ecology of Marine Protozoa. Oxford University Press, New York. CARPENTER, E. J. 1971. Annual phytoplankton cycle of the Cape Fear River Estuary, North Carolina. Chesapeake Science 12:95– 104. CHALMERS, A. G., R. G. WIEGERT, AND P. L. WOLF. 1985. Carbon balance in a salt marsh: Interactions of diffusive export, tidal deposition and rainfall-caused erosion. Estuarine, Coastal and Shelf Science 21:757–771. CHURCH, T. M. 1996. An underground route for the water cycle. Nature 380:579–580. CLARKE, J. S., C. M. HACKE, AND M. F. PECK. 1990. Geology and ground water resources of the coastal area of Georgia. Georgia Coastal Geological Survey Bulletin 113:1–106. COASTAL ASSESSMENT AND DATA SYNTHESIS SYSTEM. 1999. Physical 815 and Hydrological Characteristics of Coastal Watersheds. National Coastal Assessments Branch, Special Projects Office, National Ocean Service, National Oceanic and Atmospheric Administration, Silver Spring, Maryland. COLEMAN, V. L. AND J. M. BURKHOLDER. 1994. Community structure and productivity of epiphytic microalgae on eelgrass (Zostera marina L.) under water-column nitrate enrichment. Journal of Experimental and Marine Biology and Ecology 179:29–48. COULL, B. C. 1985. Long-term variability of estuarine meiobenthos: An 11-year study. Marine Ecology Progress Series 24:205– 213. COULL, B. C. AND S. BELL. 1979. Perspectives of meiofaunal ecology, p. 189–216. In R. J. Livingston (ed.), Ecological Processes in Coastal and Marine Systems. Plenum, New York. COUTINHO, R. 1987. Ecology of macroalgae in North Inlet estuary, S.C. Ph.D. Thesis, University of South Carolina, Columbia, South Carolina. CRITCHER, T. S. 1967. The Wildlife Values of North Carolina Estuarine Lands and Waters. North Carolina Wildlife Resources Commission. Raleigh, North Carolina. CULLITON, T. J., M. A. WARREN, T. R. GOODSPEED, D. G. REMER, C. M. BLACKWELL, AND J. J. MCDONOUGH. 1990. 50 Years of Population Change Along the Nation’s Coast, 1960–2010. National Ocean Service, National Oceanic and Atmospheric Administration, U.S. Department of Commerce, Rockville, Maryland. DAI, T. AND R. G. WIEGERT. 1996. Ramet population dynamics and net aerial primary productivity of Spartina alterniflora. Ecology 77:276–288. DAME, R. F. 1989. The importance of Spartina alterniflora to Atlantic coast estuaries. Critical Reviews in Aquatic Sciences 1:639– 660. DAME, R. F. 1996. Ecology of Marine Bivalves: An Ecosystem Approach. CRC Press, Boca Raton, Florida. DAME, R. F. AND D. M. ALLEN. 1996. Between estuaries and the sea. Journal of Experimental Marine Biology and Ecology 200:169– 185. DAME, R. F., D. CHILDERS, AND E. KOEPFLER. 1992. A geohydrologic continuum theory for the spatial and temporal evolution of marsh-estuarine ecosystems. Netherlands Journal of Sea Research 30:63–72. DAME, R. F., T. H. CHRZANOWSKI, K. BILDSTEIN, B. KJERFVE, H. MCKELLAR, D. NELSON, J. D. SPURRIER, S. STANCYK, H. STEVENSON, F. J. VERNBERG, AND R. G. ZINGMARK. 1986. The outwelling hypothesis and North Inlet, South Carolina. Marine Ecology Progress Series 33:217–229. DAME, R. F., J. D. SPURRIER, T. M. WILLIAMS, B. KJERFVE, R. G. ZINGMARK, T. G. WOLAVER, T. H. CHRZANOWSKI, H. MCKELLAR, AND F. J. VERNBERG. 1991. Annual material processing by a salt marsh-estuarine basin in South Carolina. Marine Ecology Progress Series 72:153–166. DAME, R. F., J. D. SPURRIER, AND T. G. WOLAVER. 1989. Carbon, nitrogen and phosphorus processing by an oyster reef. Marine Ecology Progress Series 54:249–256. DARDEAU, M. R., R. F. MAUDLIN, W. W. SCHROEDER, AND J. P. STOUT. 1992. Estuaries, p. 615–744. In C. T. Hackney, S. M. Adams, and W. H. Martin (eds.), Biodiversity of the Southeastern United States. Aquatic Communities. John Wiley & Sons, Inc., New York. DAY, J. W., C. A. S. HALL, W. M. KEMP, AND S. YANEZ-ARANCIBIA. 1989. Estuarine Ecology. Wiley, New York. DYNESIUS, M. AND C. NILSSON. 1994. Fragmentation and flow regulation of river systems in the northern third of the world. Science 266:753–762. EARTHINFO, INC. 1997. EarthInfo CD2 U.S. Geological Survey Daily Values. Boulder, Colorado. ELEUTERIUS, L. N. 1976. The distribution of Juncus roemerianus 816 R. Dame et al. in the salt marshes of North America. Chesapeake Science 17: 289–292. ENVIRONMENTAL PROTECTION DIVISION. 1997. Interim Strategy for Managing Salt Water Intrusion in the Upper Floridian Aquifer of Southeast Georgia. April 23, 1997. Environmental Protection Division, Georgia Department of Natural Resources, Atlanta, Georgia. EYRE, B. AND C. TWIGG. 1997. Nutrient behaviour during postflood recovery of the Richmond River Estuary, Northern NSW, Australia. Estuarine, Coastal and Shelf Science 44:311–326. FULTON, R. S. 1984. Distribution and community structure of estuarine copepods. Estuaries 7:38–50. GALLAGHER, J. L. AND R. J. REIMOLD. 1973. Tidal marsh plant distribution and productivity patterns from the sea to freshwater—A challenge in resolution and discrimination, p. 165– 183. In IV Biennial Workshop on Color Aerial Photography in Plant Sciences and Related Fields. American Society of Photography, Washington, D.C. GARDNER, L. R., W. K. MICHENER, T. M. WILLIAMS, E. R. BLOOD, B. KJERFVE, L. A. SMOCK, D. J. LIPSCOMB, AND C. GRESHAM. 1992. Disturbance effects of Hurricane Hugo and a pristine coastal landscape: North Inlet, South Carolina, USA. Netherlands Journal of Sea Research 30:249–263. GILMORE, R. G. 1995. Environmental biogeographic factors influencing ichthyofaunal diversity: Indian River Lagoon. Bulletin of Marine Science 57:153–170. GLIBERT, P. M., J. C. GOLDMAN, AND E. J. CARPENTER. 1982. Seasonal variations in the utilization of ammonium and nitrate by phytoplankton in Vineyard Sound, Massachusetts, USA. Maine Biology 70:237–249. HAINES, B. L. AND E. L. DUNN. 1985. Coastal marshes, p. 323– 347. In B. F. Chabot and H. A. Mooney (eds.), Physiological Ecology of North American Plant Communities. Chapman and Hall, New York. HAINES, E. B. 1976. Stable carbon isotope ratios in the biota, soils and tidal water of a Georgia salt marsh. Estuarine Coastal Marine Science 4:609–616. HANSON, K. AND G. MAUL. 1991. Florida precipitation and the Pacific El Niño, 1895–1989. Florida Scientist 54:160–168. HAYES, M. O. 1978. Impact of hurricanes on sedimentation in estuaries, bays, and lagoons, p. 323–346. In M. L. Wiley (ed.), Estuarine Interactions. Academic Press, New York. HEALD, E. J. 1969. The production of organic detritus in a south Florida estuary. Ph.D. Dissertation, University of Miami, Miami, Florida. HEFNER, J. M., B. O. WILLEN, T. E. DAHL, AND W. E. FRAYER. 1994. Southeast Wetlands. U.S. Department of the Interior, Fish and Wildlife Service and U.S. Environmental Protection Agency, Washington, D.C. (http://www.nwi.fws.gov/sewet/) HETTLER, JR., W. F. 1989. Nekton use of regularly flooded salt marsh cordgrass habitat in North Carolina, USA. Fishery Bulletin 56:111–118. HOERLING, M. P. AND A. KUMAR. 1997. Why do North American climate anomalies differ from one El Niño event to another? Geophysical Research Letters 24:1059–1062. HOESE, H. D. 1971. Dolphin feeding out of water in a salt marsh. Journal of Mammalogy 52:222–223. HOUSER, D. S. AND D. M. ALLEN. 1996. Zooplankton dynamics in an intertidal salt-marsh basin. Estuaries 19:659–673. HOWARTH, R. W., G. BILLEN, D. SWANEY, A. TOWNSEND, N. JAWORSKI, K. LAJTHA, J. A. DOWLING, R. ELMGREN, N. CARACO, T. JORDAN, F. BERENDSE, J. FRENEY, V. KUDEYROF, P. MURDOCH, AND Z. ZHAO-LIANG. 1996. Regional nitrogen budgets and riverine N and P fluxes for the drainages to the North Atlantic Ocean: Natural and human influences. Biogeochemistry 35:75– 139. HUMM, H. J. 1969. Distribution of marine algae along the Atlantic coast of North America. Phycologia 7:43–53. JENSEN, P. R. AND R. A. GIBSON. 1986. Primary production in three subtropical seagrass communities: A comparison of four autotrophic components. Florida Scientist 49:129–141. KAWAGUCHI, T., A. J. LEWITUS, C. M. AELION, AND H. N. MCKELLAR. 1997. Can urbanization limit iron availability to estuarine algae? Journal of Experimental Marine Biology and Ecology 213:53–69. KELLER, A. A., C. A. OVIATT, H. A. WALKER, AND J. D. HAWAK. 1999. Predicted impacts of elevated temperature on the magnitude of the winter-spring phytoplankton bloom in temperate coastal waters: A mesocosm study. Limnology and Oceanography 44:344–356. KJERFVE, B. 1989. Estuarine geomorphology and physical oceanography, p. 47–78. In J. Day, C. Hall, M. Kemp, and A. YanezArancibia, Estuarine Ecology. Wiley, New York. KJERFVE, B., J. E. GREER, AND R. L. CROUT. 1978. Low-frequency response of estuarine sea level to non-local forcing, p. 497– 513. In M. Wiley (ed.), Estuarine Interactions. Academic Press, New York. KNEIB, R. T. 1997. The role of tidal marshes in the ecology of estuarine nekton. Oceanography and Marine Biology: An Annual Review 35:163–220. KUHNEL, I., T. A. MCMAHON, B. L. FINLAYSON, A. HAINES, P. H. WHETTON, AND T. T. GIBSON. 1990. Climatic influences on streamflow variability: A comparison between Southeastern Australia and Southeastern United States of America. Water Resources Research 26:2483–2496. LE GALL, S., M. B. HASSEN, AND P. LE GALL. 1997. Ingestion of a bacterivorous ciliate by the oyster Crassostrea gigas: Protozoa as a trophic link between picoplankton and benthic suspension-feeders. Marine Ecology Progress Series 152:301–306. LEWITUS, A. J., K. C. HAYES, S. G. GRANDSEN, H. B. GLASGOW, JR., J. M. BURKHOLDER, P. M. GLIBERT, AND S. MORTON. in press. Ecological characterization of a widespread red tide in South Carolina estuaries: A newly observed phenomenon. Proceedings of the Harmful Algal Blooms 2000 9th Conference, Tasmania, Australia, February 7–11, 2000. LEWITUS, A. J., R. V. JESIEN, T. M. KANA, J. M. BURKHOLDER, H. B. GLASGOW, JR., AND E. MAY. 1995. Discovery of the ‘‘phantom’’ dinoflagellate in Chesapeake Bay. Estuaries 18:373–378. LEWITUS, A. J., E. T. KOEPFLER, AND J. T. MORRIS. 1998. Seasonal variation in the regulation of phytoplankton by nitrogen and grazing in a salt-marsh estuary. Limnology and Oceanography 43: 636–646. LEWITUS, A. J., E. T. KOEPFLER, AND R. PIGG. 2000. Use of dissolved organic nitrogen by a salt marsh phytoplankton bloom community. Archiv fur Hydrobiologie Special Issues in Advances in Limnology 55:441–456. LOMAS, M. W. AND P. M. GLIBERT. 1999. Temperature regulation of nitrate uptake: A novel hypothesis about nitrate uptake and reduction in cool-water diatoms. Limnology and Oceanography 44:556–572. LONSDALE, D. J. AND B. C. COULL. 1977. Composition and seasonality of zooplankton of North Inlet, South Carolina. Chesapeake Science 18:272–283. LUGO, A. E. AND S. C. SNEDAKER. 1974. The ecology of mangroves. Annual Review of Ecology and Systematics 5:39–64. MALLIN, M. A. 1991. Zooplankton abundance and community structure in a mesohaline North Carolina estuary. Estuaries 14: 481–488. MALLIN, M. A. 1994. Phytoplankton ecology of North Carolina estuaries. Estuaries 17:561–574. MALLIN, M. A. 2000. Impacts of industrial animal production on rivers and estuaries. American Scientist 88:24–37. MALLIN, M. A., J. M. BURKHOLDER, AND M. J. SULLIVAN. 1992. Contributions of benthic microalgae to coastal fishery yield. Transactions of the American Fisheries Society 121:691–695. MALLIN, M. A. AND H. W. PAERL. 1994. Planktonic trophic trans- Southeast Atlantic U.S. Estuaries fer in an estuary: Seasonal, diel, and community structure effects. Ecology 75:2168–2184. MALLIN, M. A., M. H. POSEY, G. C. SHANK, M. R. MCIVER, S. H. ENSIGN, AND T. D. ALPHIN. 1999. Hurricane effects on water quality and benthos in the Cape Fear Watershed: Natural and anthropogenic impacts. Ecological Applications 9:350–362. MANHEIM, F. T., R. H. MEADE, AND G. C. BOND. 1970. Suspended matter in surface waters of the Atlantic Continental Margin from Cape Cod to the Florida Keys. Science 167:371–376. MARSHALL, H. G. 1985. Phytoplankton assessment of the Duplin River, Georgia. Castanea 50:187–194. MCIVOR, C. C. AND L. P. ROZAS. 1996. Direct nekton use of intertidal salt marsh habitats: A review from the southeastern United States, p. 311–334. In K. F. Nordstrom and C. T. Roman (eds.), Estuarine Shores: Evolution, Environments, and Human Alterations. Wiley, New York. MEADE, R. H. 1969. Landward transport of bottom sediments in estuaries of the Atlantic Coastal Plain. Journal of Sedimentary Petrology 39:222–234. MEADE, R. H. 1972. Sources and sinks of suspended matter on continental shelves, p. 249–262. In D. J. P. Swift, D. B. Duane, and O. H. Pilkey (eds.), Shelf Sediment Transport: Process and Pattern. Dowden, Hutchinson and Ross, Stroudsburg. MEADE, R. H. 1982. Sources, sinks, and storage of river sediment in the Atlantic drainage of the United States. The Journal of Geology 90:235–252. MITSCH, W. J. AND J. G. GOSSELINK. 1993. Wetlands. Van Nostrand Reinhold, New York. MONTAGUE, C. L. 1997. The future of Florida’s coast. Journal of Public Interest in the Environment 1:51–59. MONTAGUE, C. L. AND H. T. ODUM. 1997. Intertidal marshes of Florida’s Gulf Coast, p. 1–33. In C. L. Coultas and Y. P. Hsieh (eds.), Ecology and Management of Tidal Marshes: A Model from the Gulf of Mexico. St. Lucie Press, Delray Beach, Florida. MONTAGUE, C. L. AND R. G. WIEGERT. 1990. Salt marshes, p. 481– 516. In R. L. Myers and J. J. Ewel (eds.), Ecosystems of Florida. University of Central Florida Press, Orlando, Florida. MOORE, W. S. 1996. Large groundwater inputs to coastal waters revealed by 226Ra enrichments. Nature 380:612–614. MULHOLLAND, P. J. AND C. R. OLSEN. 1992. Marine origin of Savannah River sediments: Evidence from radioactive and stable isotope tracers. Estuarine, Coastal and Shelf Science 34:95– 107. NATIONAL OCEANIC AND ATMOSPHERIC ADMINISTRATION. 1996. NOAA’s Estuarine Eutrophication Survey. Volume 1: South Atlantic Region. Office of Ocean Resources Conservation Assessment, Silver Spring, Maryland. NATIONAL OCEANIC AND ATMOSPHERIC ADMINISTRATION. 1997. The Deadliest, Costliest, and Most Intense United States Hurricanes of this Century. Technical Memorandum N.W.S. TPC1. Silver Spring, Maryland. NATIONAL OCEANIC AND ATMOSPHERIC ADMINISTRATION. 1999. Coastal Assessment and Data Synthesis System. Eutrophication Assessment—Regional Summary. Silver Spring, Maryland. NELSON, D. M., E. A. IRLANDI, L. R. SETTLE, M. E. MONACO, AND L. E. COSTON-CLEMENTS. 1991. Distribution and Abundance of Fishes and Invertebrates in Southeast Estuaries. Estuarine Living Marine Resources Report No. 9. National Oceanic and Atmospheric Administration/National Ocean Service Strategic Environmental Assessments Division, Rockville, Maryland. NEWELL, S. Y. 1996. Established and potential impacts of eukaryotic mycelial decomposers in marine/terrestrial ecotones. Journal of Experimental Marine Biology and Ecology 200: 187–206. ODUM, E. P. AND A. DE LA CRUZ. 1967. Particulate organic detritus in a Georgia salt marsh-estuarine ecosystem, p. 383–388. 817 In G. H. Lauff (ed.), Estuaries. American Association for the Advancement of Science, Washington, D.C. ODUM, W. E. AND E. J. HEALD. 1972. Trophic analysis of an estuarine mangrove community. Bulletine of Marine Science 22: 671–738. ODUM, W. E. AND C. C. MCIVOR. 1990. Mangroves, p. 517–548. In R. L. Myers and J. J. Ewel (eds.), Ecosystems of Florida. University of Central Florida Press, Orlando, Florida. ODUM, W. E., T. J. SMITH, J. E. HOOVER, AND C. C. MCIVOR. 1984. The Ecology of Tidal Freshwater Marshes of the United States East Coast: A Community Profile. U.S. Fish and Wildlife Service, FWS/OBS-87/17, Washington, D.C. ORLANDO, S. P., P. H. WENDT, C. J. KLEI, M. E. PATTILLO, K. C. DENNIS, AND G. H. WARD. 1994. Salinity Characteristics of South Atlantic Estuaries. National Oceanic and Atmospheric Administration, Ocean Resource Conservation and Assessment, Silver Spring, Maryland. PAERL, H. W. 1997. Coastal eutrophication and harmful algal blooms: Importance of atmospheric deposition and groundwater as ‘‘new’’ nitrogen and other nutrient sources. Limnology and Oceanography 42:1152–1165. PATULLO, J., W. H. MUNK, R. REVELLE, AND E. STRONG. 1955. The seasonal oscillation in sea level. Journal of Marine Research 14:88–156. PAULY, D. AND V. CHRISTENSEN. 1995. Primary production required to sustain global fisheries. Nature 374:255–257. PENHALE, P. A. 1977. Macrophyte-epiphyte biomass and productivity in an eelgrass (Zostera marina L.) community. Journal of Experimental Marine Biology and Ecology 26:211–224. PETERS, D. S. AND W. E. SCHAAF. 1991. Empirical model of the trophic basis for fishery yield in coastal waters of the Eastern USA. Transactions of the American Fisheries Society 120:459–473. PETERSON, B. J. AND R. W. HOWARTH. 1987. Sulfur, carbon, and nitrogen isotopes used to trace organic matter flow in the saltmarsh estuaries of Sapelo Island, Georgia. Limnology and Oceanography 32:1195–1213. PHILANDER, S. G. H. 1990. El Niño, La Niña and the Southern Oscillation. Academic Press, New York. PINCKNEY, J. L., H. W. PAERL, M. HARRINGTON, AND K. HOWE. 1998. Annual cycles of phytoplankton community structure and bloom dynamics in the Neuse River Estuary, NC. Marine Biology 131:371–381. PINCKNEY, J. L. AND R. G. ZINGMARK. 1993a. Modeling the annual production of intertidal benthic microalgae in estuarine ecosystems. Journal of Phycology 29:396–407. PINCKNEY, J. L. AND R. G. ZINGMARK. 1993b. Biomass and production of benthic microalgal communities in five typical estuarine habitats. Estuaries 16:887–897. POMEROY, L. R. 1959. Algal productivity in salt marshes in Georgia. Limnology and Oceanography 4:386–397. POMEROY, L. R., W. DARLEY, E. DUNN, J. GALLAGHER, E. HAINES, AND D. WHITNEY. 1981. Primary production, p. 39–67. In L. R. Pomeroy and R. G. Wiegert (eds.), The Ecology of a Salt Marsh. Springer-Verlag, New York. POMEROY, L. R., H. H. HASKIN, AND R. A. RAGOTZKIE. 1956. Observations on dinoflagellate blooms. Limnology and Oceanography 1:54–60. POMEROY, L. R., L. R. SHENTON, R. D. H. JONES, AND R. J. REIMOLD. 1972. Nutrient flux in estuaries. Nutrients and Eutrophication. American Society of Limnology and Oceanography Special Symposium 1:274–291. ROMAN, M. R., M. R. REEVE, AND J. L. FROGGAT. 1983. Carbon production and export from Biscayne Bay, Florida. Temporal patterns in primary production, seston, and zooplankton. Estuarine, Coastal and Shelf Science 17:45–59. ROPELEWSKI, C. F. AND M. S. HALPERT. 1986. North American precipitation and temperature patterns associated with the El 818 R. Dame et al. Niño/Southern Oscillation (ENSO). Monthly Weather Review 114:2352–2362. SANDERS, R. W., D. A. CARON, AND U. G. BERINGINGER. 1992. Relationships between bacteria and heterotrophic nanoplankton in marine and fresh waters: An inter-ecosystem comparison. Marine Ecology Progress Series 86:1–14. SCHUBAUER, J. P. AND D. S. HOPKINSON. 1984. Above and belowground emergent macrophyte production and turnover in a coastal marsh ecosystem, Georgia. Limnology and Oceanography 29:1052–1065. SERVICE, S. K. AND R. E. FELLER. 1992. Long-term trends of subtidal macrobenthos in North Inlet, South Carolina. Hydrobiologia 231:13–40. SHERR, B. F., E. B. SHERR, AND J. MCDANIEL. 1992. Effect of protistan grazing on the frequency of dividing cells in bacterioplankton assemblages. Applied and Environmental Microbiology 58:2381–2385. SHERR, B. F., E. B. SHERR, AND C. PEDROS-ALIO. 1989. Simultaneous measurement of bacterioplankton production and protozoan bacterivory in estuarine water. Marine Ecology Progress Series 54:209–219. SHERR, E. B., B. F. SHERR, R. D. FALLON, AND S. Y. NEWELL. 1986. Small, aloricate ciliates as a major component of the marine heterotrophic nanoplankton. Limnology and Oceanography 33: 177–183. SHERR, E. B., B. F. SHERR, AND J. MCDANIEL. 1991. Clearance rates of , 6 mm fluorescently labeled algae (FLA) by estuarine protozoa: Potential grazing impact of flagellates and ciliates. Marine Ecology Progress Series 69:81–92. SIGMON, D. E. AND L. B. CAHOON. 1997. Comparative effects of benthic microalgae and phytoplankton on dissolved silica fluxes. Aquatic Microbial Ecology 13:275–284. STEVENSON, J. C., L. G. WARD, AND M. S. KEARNEY. 1986. Vertical accretion in marshes with varying rates of sea level rise, p. 241–259. In D. A. Wolfe (ed.), Estuarine Variability. Academic Press, Orlando. STEVENSON, J. C., L. G. WARD, AND M. S. KEARNEY. 1988. Sediment transport and trapping in marsh systems: Implications of tidal flux studies. Marine Geology 80:37–59. SULLIVAN, M. J. AND C. A. MONCREIFF. 1990. Edaphic algae are an important component of salt marsh food-webs: Evidence from multiple stable isotope analyses. Marine Ecology Progress Series 62:149–159. SUN, H. AND D. J. FURBISH. 1997. Annual precipitation and river discharges in Florida in response to El Niño- and La Niña-sea surface temperature anomalies. Journal of Hydrology 199:74– 87. TESTER, P., M. GEESEY, C. GUO, H. PAERL, AND D. MILLIE. 1995. Evaluating phytoplankton dynamics in the Newport River estuary (North Carolina, USA) by HPLC-derived pigment profiles. Marine Ecology Progress Series 124:237–245. TESTER, P. A., R. STUMPF, F. M. VUKOVICH, P. K. FOWLER, AND J. T. TURNER. 1991. An expatriate red tide bloom: Transport, distribution and persistence. Limnology and Oceanography 36: 1053–1061. THAYER, G. W. 1971. Phytoplankton production and the distribution of nutrients in a shallow unstratified estuarine system near Beaufort, N.C. Chesapeake Science 12:240–253. THAYER, G. W., D. E. HOSS, M. A. KJELSON, W. F. HETTLER, AND M. W. LACROIX. 1974. Biomass of zooplankton in the Newport River Estuary and the influence of postlarval fishes. Chesapeake Science 15:9–16. THAYER, G. W., D. A. WOLFE, AND R. B. WILLIAMS. 1975. The impact of man on seagrass systems. American Scientist 63:288– 296. TISSUE, T., S. LEWIS, H. WOOD, J. KENDER, AND J. K. ABOH. 1994. Effects of Hurricane Hugo on sediment quality and distribution in the Charleston harbor estuary, USA, p. 61–66. In K. R. Dyer and R. J. Orth (eds.), Changes in Fluxes in Estuaries: Implications from Science to Management. Olsen and Olsen, Fredensborg, Denmark. TURNER, R. E. 1976. Geographic variations in salt marsh macrophyte production: A review. Contributions in Marine Science (Texas) 20:47–68. VALIELA, I., J. M. TEAL, S. D. ALLEN, R. V. VAN ETTEN, D. GOEHRINGER, AND S. VOLKMANN. 1985. Decomposition in salt marsh ecosystems: The phases and major factors affecting disappearance of aboveground organic matter. Journal of Experimental Marine Biology and Ecology 89:29–54. VAN DOLAH, R. F. AND G. S. ANDERSON. 1991. Effects of Hurricane Hugo on salinity and dissolved oxygen conditions in Charleston Harbor estuary. Journal of Coastal Research 8:83–94. VERITY, P. G., J. A. YODER, J. S. BISHOP, J. R. NELSON, D. B. CRAVEN, J. O. BLANDON, C. Y. ROBERTSON, AND C. R. TRONZO. 1993. Composition, productivity, and nutrient chemistry of a coastal ocean planktonic food web. Continental Shelf Research 13:741–776. VERNBERG, F. J. 1993. Salt-marsh processes: A review. Environmental Toxicology and Chemistry 12:2167–2195. VERNBERG, F. J., W. B. VERNBERG, E. BLOOD, A. FORTNER, M. FULTON, H, MCKELLAR, W. MICHENER, G. SCOTT, T. SIEWICKI, AND K. EL FIGI. 1992. Impact of urbanization on high-salinity estuaries in the Southeastern United States. Netherlands Journal of Sea Research 30:239–248. VIRNSTEIN, R. W., W. G. NELSON, F. G. LEWIS III, AND R. K. HOWARD. 1984. Latitudinal patterns in seagrass epifauna: Do patterns exist and can they be explained? Estuaries 7:310–330. VOGEL, R. L., B. KJERFVE, AND L. R. GARDNER. 1996. Inorganic sediment budget for North Inlet salt marsh, South Carolina, U.S.A. Mangroves and Salt Marshes 1:23–35. WARD, L. G. 1981. Suspended-material transport in marsh tidal channels, Kiawah Island, South Carolina. Marine Geology 40: 139–154. WEINSTEIN, M. P. 1979. Shallow marsh habitat as primary nurseries for fishes and shellfish, Cape Fear River, North Carolina. Fishery Bulletin 77:339–357. WETZ, M., A. J. LEWITUS, E. T. KOEPFLER, AND K. C. HAYES. 1999. Seasonal and spatial variation within the microzooplankton community of North Inlet estuary, SC, p. 1263–1267. In R. D. Yearout (ed.), Proceedings National Conference on Undergraduate Research 1999, Volume 4. The University of North Carolina at Asheville, Ashville, North Carolina. WILKINSON, M. 1980. Estuarine benthic algae and their environment: A review, p. 425–486. In J. H. Price, D. E. G. Irvine and W. F. Farnham (eds.), The Shore Environment, Volume 2. Academic Press, New York. WINDOM, H. L., W. J. NEAL, AND K. C. BECK. 1971. Mineralogy of sediments in three Georgia estuaries. Journal of Sedimentary Petrology 41:497–504. WOODMANSEE, R. A. 1958. The seasonal distribution of the zooplankton off Chicken Key in Biscayne Bay, Florida. Ecology 39: 247–262. ZEITZSCHEL, B. 1990. Zoogeography of marine protozoa: An overview emphasizing distribution of planktonic forms, p. 139–185. In G. M. Capriulo (ed.), Ecology of Marine Protozoa. Oxford University Press, New York. ZIEMAN, J. C. 1975. Tropical seagrass systems and pollution, p. 63–74. In E. J. F. Wood and R. E. Johannes (eds.), Tropical Marine Pollution. Elsevier, New York. ZIEMAN, J. C. 1982. The Ecology of the Seagrasses of South Florida: A Community Profile. U.S. Fish and Wildlife Service, Office of Biological Services, Washington, D.C. ZIEMAN, J. C. AND R. WETZEL. 1980. Methods and rates of productivity in seagrasses, p. 87–116. In R. C. Phillips and C. P. McRoy (eds.), Handbook of Seagrass Biology. Garland STMP Press, New York. Southeast Atlantic U.S. Estuaries SOURCES OF UNPUBLISHED MATERIALS MORAN, M. A. personal communication. Department of Marine Sciences, University of Georgia, Athens, Georgia 30602. MORTON, S. L. personal communication. Marine Biotoxins Program, National Ocean Service, Charleston Laboratory, 219 Fort Johnson Road, Charleston, South Carolina 29422. 819 NORTH CAROLINA DEPARTMENT OF WATER QUALITY. 1996. Cape Fear River Basinwide Water Quality Management Plan. North Carolina Division of Water Quality, Water Quality Section, P.O. Box 29535, Raleigh, North Carolina. Received for consideration, November 16, 1998 Accepted for publication, September 22, 2000