HIV-1 Antibody Confirmation by Western Blot
advertisement

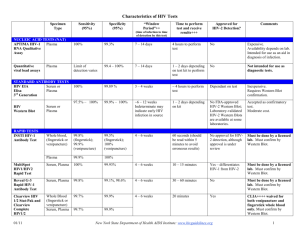
Lab Dept: Serology Test Name: HIV 1 ANTIBODY CONFIRMATION BY WESTERN BLOT General Information Lab Order Codes: HIVCO Synonyms: W. Blot Anti-HIV; HTLV III; AIDS; Aquired Immune Deficiency Syndrome; HIV1 Antibody CPT Codes: 86689 – Antibody; HTLV or HIV antibody, confirmatory test Test Includes: HIV-1 Western Blot (WB) analysis reported as Negative, Positive or Indeterminate. See HIV Serologic Screening Algorithm for more information. Logistics Test Indications: Supplemental serologic testing for HIV-1 infection in patients with indeterminate or reactive HIV-1 antibody results (regardless of HIV-2 antibody result) by FDA-approved HIV-1/2 antibody differentiation test. Lab Testing Sections: Serology - Sendouts Referred to: Mayo Medical Laboratories (MML Test: HV1WB) Phone Numbers: MIN Lab: 612-813-6280 STP Lab: 651-220-6550 Test Availability: Daily, 24 hours Turnaround Time: 1 – 3 days Special Instructions: N/A Specimen Specimen Type: Blood Container: Red top tube Draw Volume: 4.8 mL (Minimum: 1.2 mL) blood Processed Volume: 1.6 mL (Minimum: 0.4 mL) serum Collection: Routine venipuncture Special Processing: N/A Patient Preparation: Lab Staff: Centrifuge specimen, remove serum aliquot and send specimen in a plastic, screw top tube. Send specimen frozen. Forward promptly. Sample Rejection: Mislabeled or unlabeled specimens, specimens other than serum Interpretive Reference Range: Negative. Critical Values: N/A Limitations: The US Association of Public Health Laboratories recommends verification of all first-time positive confirmatory test results prior to the diagnosis of HIV infection. A second serum should be obtained from the patient and submitted for repeat testing to verify all positive results. Positive HIV antibody WB results in infants of <18 months of age and born to HIV-infected mothers may indicate passive transfer of maternal HIV antibodies. Serologic tests (screening, supplemental or confirmatory) cannot distinguish between active neonatal HIV infection and passive transfer of maternal HIV antibodies in infants during the postnatal period (up to 18 months). Diagnosis of HIV infection in newborns and infants should be made by virologic tests such as detection of HIV RNA or HIV proviral DNA. This test should be ordered only on sera that are either indeterminate or reactive for HIV-1 antibodies (regardless of the HIV-2 antibody result) by FDA approved HIV-1/2 antibody differentiation tests. Although hemolyzed serum specimens are acceptable for testing, this assay is not FDA-approved for testing cadaveric serum specimens. Performance characteristics have not been established for the following specimen types: Cadaveric specimens, Specimens containing particulate matter Methodology: HIV-1 WB: Western Blot References: Mayo Medical Laboratories November 2014 Updates: 4/6/2004: Test moved from Memorial Blood Center of Minneapolis to Mayo Medical Laboratories. Note: Test now reflexes to supplemental/confirmatory testing (with additional charges) when indicated by reactive findings. 9/27/2010: Specimen requirement updates.