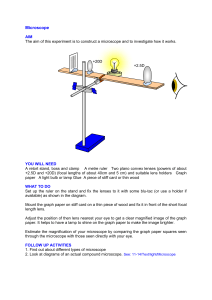
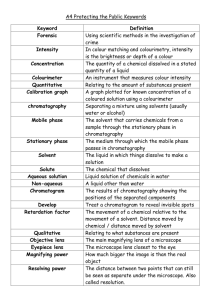
Biology 120 Laboratory Manual
advertisement

Biology 120 Laboratory Manual Yok-Keng Graal ,Todd Harper, and Greg Doheny Columbia College Biology 120 Laboratory Manual 1 Contents INTRODUCTION .................................................................................................................................................. 4 Introduction: ............................................................................................................................................................. 4 Policy on absences from the lab sessions: ........................................................................................ 4 Policy on late lab reports: ......................................................................................................................... 4 Policy on plagiarism: ................................................................................................................................... 4 How the Lab Reports work: ...................................................................................................................... 5 Laboratory Conduct: .................................................................................................................................... 5 Policy on Food and Drink: ......................................................................................................................... 5 Laboratory Clean Up: ................................................................................................................................... 6 Leaving the Lab:.............................................................................................................................................. 6 Laboratory Safety: ......................................................................................................................................... 6 Laboratory Exercise 1: Introduction to Microscopy.................................................................................... 8 Part A: Pre-Lab Quiz Questions. ............................................................................................................. 8 Part B: Lab Protocols. .................................................................................................................................. 9 EXERCISE I: Parts of the Compound Microscope .......................................................................... 11 EXERCISE II: Using the Compound Microscope to Estimate the Sizes of Plant Cells. ...... 12 EXERCISE III: Estimating the Size of a Human Erythrocyte. ..................................................... 13 EXERCISE IV: Estimating the resolution limit for the compound microscope...................... 13 EXERCISE V: Using the oil immersion lens. ................................................................................... 14 EXERCISE VI: Using the dissecting microscope. ........................................................................... 16 Part C: To Be Handed In Next Session. ............................................................................................. 17 Laboratory Exercise 2: Cells and Osmosis ................................................................................................. 20 Part A: Pre-Lab Quiz Questions. (2.5 points) ............................................................................... 20 Part B: Pre-Lab Exercise. ........................................................................................................................ 20 Part B: Lab Protocols. ............................................................................................................................... 25 Part C: To Be Handed In Next Session .............................................................................................. 27 Laboratory Exercise 3: Characteristics of Enzymes ................................................................................. 28 Part A: Pre-Lab Quiz Questions (2.5points).................................................................................. 28 Part B: Pre-Lab Exercise. This section to be handed in at the start of class (2.5 points) .............................................................................................................................................................................. 28 Part B: Lab Protocols. ............................................................................................................................... 31 2 Part C: To Be Handed In Next Session. ............................................................................................. 34 Laboratory Exercise 4: Photosynthetic Plant Pigments ........................................................................... 35 Part A: Pre-Lab Quiz Questions. .......................................................................................................... 35 Part B: Lab Protocols. ............................................................................................................................... 36 Part C: To Be Handed In Next Session. ............................................................................................. 40 Laboratory Exercise 5: Plant Anatomy ........................................................................................................ 41 Part A: Pre-Lab Quiz Questions. .......................................................................................................... 41 Part B: Lab Protocols. ............................................................................................................................... 42 Part C: To Be Handed In Next Session. ............................................................................................. 47 Laboratory Exercise 6: Digestive Systems .................................................................................................. 48 Part A: Pre-Lab Quiz Questions. .......................................................................................................... 48 Part B: Lab Protocols. ............................................................................................................................... 49 Part C: To Be Handed In Next Session. ............................................................................................. 54 Laboratory Exercise 7: Circulatory Systems .............................................................................................. 56 Part A: Pre-Lab Quiz Questions. .......................................................................................................... 56 Part B: Lab Protocols. ............................................................................................................................... 57 Part C: To Be Handed In Next Session. ............................................................................................. 64 Laboratory Exercise 8: The Respiratory System ....................................................................................... 66 Part A: Pre-Lab Quiz Questions. .......................................................................................................... 66 Part B: Lab Protocols. ............................................................................................................................... 67 Part C: To Be Handed In Next Session. ............................................................................................. 71 Laboratory Exercise 9: The Renal and Reproductive Systems ............................................................. 72 Part A: Pre-Lab Quiz Questions. .......................................................................................................... 72 Part B: Lab Protocols. ............................................................................................................................... 73 Part C: To Be Handed In Next Session. ............................................................................................. 77 3 INTRODUCTION Introduction: Welcome to the Biology 120 Laboratory, where you will complete 13 lab sessions. In the first lab session (Week 1) you will write a multiple choice pre-test. This test is not for credit, and its purpose is merely to determine what level of knowledge the class has at the start of the course. This will be followed by nine lab exercises, and final laboratory exam. Each lab exercise must be completed and submitted at the start of the next lab session. The lab reports are worth a cumulative 10% of your mark. The lab exam is worth 10% of your mark. Policy on absences from the lab sessions: Each lab is worth 10 points, for a cumulative 10% of your grade. Attendance at lab sessions is mandatory. If you miss a lab I cannot accept your report for that lab, and you will get a mark of zero for that lab. If you miss a lab due to illness you must provid a doctor’s note, which includes contact information for the doctor. I will then calculate your lab mark based on the labs you did not miss. Policy on late lab reports: The report for each lab is due at the beginning of the next lab session. When you enter the lab, leave your report on the front bench before sitting down. Each lab is worth 10 points, and I will subtract 2 points if the lab is not submitted at the beginning of the lab. (For example, if you submit it at the end of the lab, or later the same day.) I cannot accept lab reports on other days, unless you were absent from the lab for medical reasons. Again, I will require a doctor’s note. Policy on plagiarism: In layman’s terms, plagiarism is the act of submitting somebody else’s work while pretending that it is your own. As stated in the Columbia College calendar: “Plagiarism, the presentation of another’s words, thoughts or inventions as one’s own, is regarded as a grave offense in all courses at Columbia College.” Plagiarism is taken very seriously by all universities and colleges (not just Columbia College), and is generally punished with an “F” grade for the course, and/or expulsion from the college. One of the reasons for the severe punishments associated with plagiarism is that plagiarism, unlike other forms of cheating, is very easy to prove. If you submit a written work to us, and we can find exactly the same words written someplace else (on the internet, for example, or in a book, or in another student’s lab report) we have definitive proof that you copied it. Students will often work on lab reports together, or as a group, and then submit lab reports which contain exactly the same words as the lab reports of their friends. They are usually unaware that this constitutes plagiarism, when, in fact, it does. Ideally, you should work on your lab report alone, but I realize that it is impractical to forbid you from working on your lab reports with your 4 friends. However, if you work in groups, please do not use the same wording. If you do, this would be a clear and provable example of plagiarism. How the Lab Reports work: You must read and prepare for each lab before coming to the lab. If you come to the lab unprepared, and try to read the instructions as you go along you will not have enough time to finish. For some labs, you will actually be expected to complete an exercise beforehand, and submit it when you arrive. You will also be given a quiz at the beginning of each lab. The quiz is worth 5 marks, and the lab report is worth 5 marks. The quiz will contain questions about what is in the lab, and some of the answers to the quiz questions can be found in the lab protocols. So, it is definitely in your best interest to read the lab protocols before coming to the lab. The questions you may be asked on the quiz are given to you in advance, and can be found at the beginning of each lab. Each lab may consist of up to four parts: A. An exercise that you must complete before coming to the lab, and which you must submit when you arrive. (Lab 2 only.) B. A lab quiz, given at the beginning of the lab period. In some cases, the answers to the questions can be found within the lab protocol. C. A section containing the PROTOCOLS (or instructions) for what you’re supposed to be doing in the lab. As you go through the lab, you should write notes in your lab book, and use these notes later on to fill in Section D. And, D. A section that you fill out on your computer, and submit to me the following week. You will need to buy a lab notebook of some sort. The notebook is for you to make notes in as you do the labs. You will need these notes and observations to fill out the lab questions that you will be submitting the following week. You are responsible for printing out the sections of the lab that you will need in order to complete the lab. You will then type out your results and conclusions on your own computer (or one of the computers in the College) in Section D, and submit it to me the following week. Each lab report is worth 10 marks. The marks are distributed between these sections as indicated. As mentioned above, not every lab report will have all four sections. Some may have only two. Laboratory Conduct: You are not permitted to play, joke around, or otherwise behave in an unsafe manner in the laboratory. Remember, this is a potentially dangerous environment. DO NOT WRITE ON THE LAB BENCH. Keep the lab bench clean. Policy on Food and Drink: Absolutely NO FOOD OR DRINK of any kind is allowed in the lab! This includes water bottles. If you really need to take a drink, you must go outside the lab. This is a stardard policy for all college and university Chemistry and Biology labs, and is the policy for most Physics labs as well. 5 Laboratory Clean Up: After each laboratory experiment, the students are expected to clean up their work bench, and any equipment they used. This is particularly true of the dissection instruments (forceps, scissors, scalpels etc.), which must be washed and put back into the proper containers. Prepared microscope slides must be put back in the correct box. Glass slides that you have prepared should be washed and left to dry on a paper towel by the sink. Slide cover slips must go in the yellow ‘sharps discard’ (your instructor will show you where this is). Leaving the Lab: You must ask permission to leave the lab, so that your instructor can confirm that you have finished everything. More importantly, you may not leave the lab early, and expect your lab partners to either complete the experiment, or clean up the mess. Laboratory Safety: The Biology Lab is generally not as dangerous as the Chemistry Lab, but it still contains many hazards, such as open flames, sharp instruments, and toxic chemicals, which you must be careful of. The following safety precautions must be observed: 1. Long Hair: Long hair (including bangs) must be pulled back and tied into a pony tail. (It can easily catch fire if we are using an open flame, or fall inside the body of a rat we are dissecting.) 2. Open-Toed Shoes: Open-toed shoes are discouraged, as sharp objects (scalpels, scissors and other blades) or hazardous chemicals can fall onto unprotected toes and cause injury. 3. Dissection Tools: We use a variety of sharp cutting tools to do dissections in the Biology 120 lab. Be very careful when handling these instruments. Hold them tightly and don’t drop them. Exercise due caution when handling them. Not only can they cause cuts, but if you are using them to dissect animals (like rats) they can also cause infection by bacteria, viruses or fungi. 4. SHARPS: Any disposable sharp objects (needles, blades, microscope cover slips) are collectively called ‘sharps,’ and must be disposed of, carefully, in a yellow container called a ‘sharps discard.’ The sharps discards are located at the back of the lab on the side bench. Note that microscope coverslips have extremely sharp corners, even without being broken! Therefore, microscope cover slips must carefully be discarded in the sharps container after use. Microscope slides can be washed and re-used, but cover slips must go in the sharps container. 5. Broken Glass: If your break glass, do not attempt to clean it up yourself! Tell your instructor, and he/she will clean it up. Large amounts of broken glass go in the broken glass bin in the Chemistry Lab. 6 6. Fire Extinguisher: There is only one fire extinguisher in the lab, and it is located on the side of the front bench. If you start or see a fire, inform the instructor immediately. 7. Eye Wash Station: There is no eye wash station in the Biology Lab. If you contaminate your eyes with chemicals or other materials, have your lab partner inform the instructor immediately. The instructor will then take you next door to the Chemistry lab, where the eye wash station is located. 8. Emergency Shower: There is no emergency shower in the Biology Lab. If you spill a large quantity of toxic chemicals on yourself, have your lab partner inform the instructor immediately. The instructor will then take you next door to the Chemistry Lab, where the emergency shower is located. 7 LABORATORY EXERCISE I: MICROSCOPY Laboratory Exercise 1: Introduction to Microscopy In this lab you will learn how to use the compound microscope and the dissection microscope. In addition, you will also learn how and when to use the oil immersion lens on the compound microscope. Part A: Pre-Lab Quiz Questions. (5 points) Lab 1 Quiz Questions 1. What is the main difference between a compound microscope and a dissecting microscope? 2. What is an ocular lens? 3. What is an objective lens? 4. What is the “stage” of a compound microscope? 5. Which is the only lens on a compound microscope which is meant to be used with immersion oil? 6. If the ocular lens of a compound microscope magnifies 10X, and you are using the 4X objective lens, what is the TOTAL magnification of the image? 7. Why do you always focus a slide in a compound microscope by first looking from the side, and raising the stage up as close to the objective lens as is possible without touching it, and THEN looking down the ocular lens, and focusing the slide by moving the stage DOWNWARDS? What are you trying to avoid? 8 LAB 1 PROTOCOLS Part B: Lab Protocols. This section tells you what you’re supposed to do in this lab. Make notes in your own lab book as you go along. The microscope is one of the most important tools available to a biologist. As a biology student you must learn how to handle and use a microscope. We will be using two types of microscopes in this lab, the compound microscope and the dissecting microscope. The compound microscope uses several lenses, and gives a high degree of magnification. With a compound microscope, the specimen you wish to view must be cut into thin slices and mounted on to glass slides, so that the light can pass through the specimen. A dissecting microscope has a lower degree of magnification, and light does not pass through the specimen. Handling the microscope: Your instructor will demonstrate how to hold and carry a microscope. Always hold the microscope by the arm, and always place one hand under the base when lifting a microscope. The reason for placing your hand under the base is that the light source (light bulb) is located there, and if the screws which hold it in place are faulty, the bulb will fall out and break. Also, do not tilt the microscope when carrying it, because the ocular lenses may fall out and break. Unlike the other lenses, the ocular lenses are not screwed on to the microscope. They are held in place by gravity. Your instructor will show you the difference between a compound and a dissecting microscope. Each student should have their own compound scope, and each bench should have at least two dissecting scopes. Place them up on the bench for you to use. Each bench should also have a desk lamp, which should also be placed up on the bench. The desk lamp is for use with the dissecting scope. Parts of the compound microscope: 1. OCULAR LENSES: these are the lenses where your eyes go. An ocular lens is sometimes called an ‘eyepiece.’ Note that they are held in place by gravity, and are not screwed on to the microscope. Carefully remove one of them and look through it. Notice that it acts as a magnifying glass. Write down the magnification power of the ocular lens, and then replace it. Note also that the two ocular lenses can be moved apart to fit your interpupilary distance. Move them apart until both of your eyes can look down the ocular lenses at once. 2. BODY: the body is just a tube where light travels from the objective lenses to the ocular lens. 3. NOSEPIECE: This is a rotating turret that allows you to switch from one objective lens to another. 9 4. OBJECTIVE LENSES: There are four lenses, called objective lenses, attached to the nosepiece. Write down the magnification power of each in the space below. Also indicate which one is the OIL IMMERSION lens. This lens, and only this lens is meant to be used with immersion oil. DO NOT GET OIL ON ANY OF THE OTHER LENSES! Also, indicate which lens is the shortest/smallest. Note that the total magnification will be the product (multiplication) of the ocular and the objective lens. Therefore, if you’re viewing through the 4X objective lens, the total magnification will be 40X, because the ocular lens is a 10X lens. (4x10=40) 5. STAGE: flat surface that holds the slide in place. Notice that there is a pin which is intended to hold the slide in place against two numbered rulers. Two knobs located below the stage allow you to move the stage around, and the rulers allow you to note the coordinates of any interesting things you might see on the slide. Make sure you know how to position a slide properly inside this clip, and read the coordinate numbers off the rulers. 6. CONDENSER: This is a lens located below the stage that focuses the light from the light source. (Proper focusing of the condenser lens ensures that the light rays are parallel when passing through the slide.) Ideally, the condenser should be as close to the slide as possible. 7. CONDENSER FOCUS KNOB: This knob moves the condenser up or down in order to focus the light from the light source. 8. CONDENSER DIAPHRAGM: This is a lever that opens or closes the adjustable hole in the condenser, letting more or less light through it. If your image is hard to focus (too blurry) it might be because the condenser diaphragm is open too far; and if your image is too dark, it might be because it is closed too far. 9. LIGHT SOURCE: This is a special (and expensive) light bulb used to illuminate the slide. Some versions of the compound microscope use a mirror instead of a light bulb. 10. LIGHT SOURCE ADJUSTMENT KNOB: this knob is used to adjust the intensity of the light coming from the light source. You should keep the intensity as low as possible to avoid burning the retinas of your eyes. 11. COARSE and FINE FOCUS KNOBS: Two knobs located below the stage allow you to adjust the focus on your microscope. Note that they do this by raising or lowering the stage, rather than the lenses. The course focus knob raises or lowers the stage a great deal with each turn, while the fine focus knob only raises or lowers it a little with each turn. Note that on some microscopes the fine focus knob is located WITHIN the course focus knob, and on other microscopes the two knobs are separate. Make sure you know which is which. 10 12. BASE: This is the part of the microscope that sits on the bench, and contains the light source. Write down the four magnification powers of the four objective lenses, and indicate which one is the OIL IMMERSION lens. Also, indicate which lens is the SHORTEST. EXERCISE I EXERCISE I: Parts of the Compound Microscope Label the following parts of the compound microscope. 11 EXERCISE II EXERCISE II: Using the Compound Microscope to Estimate the Sizes of Plant Cells. Some general rules for using a microscope: 1. When plugging the microscope in, or unplugging it, always hold the plug itself. Do not pull on the cord, because it may detach from the plug and give you an electric shock. 2. Turn the level of illumination down as low as possible. Do not use more light than is necessary to see the specimen. Using too much light may damage your eyes. 3. You will notice that when you move from a lower magnification to a higher magnification you will have to increase the intensity of the light. This is normal. 4. ALWAYS focus a slide by LOWERING, rather than raising the stage. Look through your microscope, and learn which way to turn the coarse and fine focus knobs in order to LOWER the stage while you’re looking through it. (Note: it may be different on different microscopes! So, before using ANY microscope, make sure you know which way to turn the knobs in order to lower the stage.) 5. Focus a slide by looking to the SIDE of the microscope (not down the eyepieces), and raising the stage until the slide almost touches the lens. Then look through the eyepieces, and focus the slide by LOWERING the stage. The reason for this is because, if you focus by raising the stage, you might actually raise the slide right up to the lens and BREAK both the slide and the lens. (Be very careful not to break microscope lenses. They cost about $300 each, and if you break one you will have to buy the College a new one.) With these microscopes the lowest power objective lens is 4X, and it is short enough that the slide will never touch it, no matter how high you raise it. That is not true for the other lenses. So, when you move from one lens to another by turning the nosepiece, always look from the side to see whether the slide is about to touch the lens or not. Make sure that it doesn’t! 6. When you are viewing a slide for the first time, start with the lowest power magnification (4X in this case) and then move to a higher magnification by rotating the nosepiece. (Again, always look from the side while you do this, to make sure the next lens isn’t going to touch the slide.) Compound microscopes use a set of parfocal lenses. Parfocal lenses (in theory) all have the same focal distance, so that if you focus the slide for one lens, the others should be in focus also. Therefore, when you move to the next lens, you shouldn’t have to focus the slide again. At least not in theory. In reality, you may have 12 to make a SLIGHT adjustment with the fine focus knob when you move to the next lens. But at last you won’t have to change the focus very much. PROTOCOL: Estimating the size of a plant cell: 1. Obtain a ruler and a slide of a plant stem from the back of the lab. (Be sure to put the slide back in the correct box when you’re finished with it!) 2. Put the ruler under the 4X lens, focus it, and estimate how many millimeters in diameter the circular field of view is. Write down this number. 3. Rotate the nosepiece to the 40X lens. Can you see any of the marks on the ruler? Probably not, because the diameter of the field of view at 400X is only one tenth what it is at 40X, and therefore it is smaller than one millimeter. Please calculate how large the field of view is when you’re using the 4X, 10X, 40X, and 100X objective lenses. 4. Remove the ruler, and put the plant stem slide into the microscope. 5. Under the 40X lens, estimate how many plant cells it would take to get from one side of the field of view to the other. Using this estimate, and your estimate of the diameter of the field of view, estimate how big a plant cell is. 6. Put your plant cell slide back in the correct box. EXERCISE III EXERCISE III: Estimating the Size of a Human Erythrocyte. Having estimated the size of a plant cell, we’re going to repeat the same protocol with a human red blood cell (an erythrocyte). Obtain a human blood cell slide from the back of the lab, and repeat the same procedure you used above. (Don’t forget to return the slide to the right box.) These slides are stained with a stain called Wright’s Stain, which stains the red blood cells pink, and the white blood cells blue. As you will learn later in the course, the white blood cells are part of the body’s immune system. Only about 1 in every 100 blood cells is a white blood cell. The red blood cells are slightly smaller than the white blood cells. EXERCISE IV EXERCISE IV: Estimating the resolution limit for the compound microscope. 13 The Resolution Limit of a microscope is defined as the smallest distance that two objects can be separated by, and still be seen as two separate objects. For example, if the resolution of a microscope is one micron, this means that two objects that are separated by a distance of less than one micron will appear as a single object. Based on what you can see down the microscope, make an educated guess about what the resolution limit of a compound microscope is at 400X magnification. EXERCISE V EXERCISE V: Using the oil immersion lens. A process called ‘oil immersion’ is used with the 100X objective lens. In order to magnify an image, the light rays must be bent apart, or ‘refracted.’ Something called the ‘Refractive Index’ (RI) is a measure of how well light rays can be refracted when passing through a specific medium (substance). As it happens, the refractive index of air is too low to use for very high magnifications, and the image will not be very good if the light from the slide must pass through air before getting to the lens. To correct for this, we want to place something with a higher RI between the slide and the lens. In this case, a special oil is used called ‘immersion oil.’ Immersion oil has a higher RI than air. Biologists usually switch to oil immersion microscopy when they’re looking at smaller cells, like bacterial cells. For example, microbiologists are biology specialists who work mainly with bacteria; and bacterial cells are generally less than one tenth the size of animal or plant cells. Thus, if you’re interested in becoming a microbiologist, you should get used to using oil immersion. In this exercise, you will be looking at bacteria. Some things to consider when using oil immersion microscopy: 1. Immersion oil should only be used with the 100X lens, and should not touch any other lens. 2. If you are having trouble getting your image to focus with a lower power lens (ie-the image is always ‘fuzzy’ when you’re looking through the 10X lens), it might be because somebody else got immersion oil on the wrong lens. 3. You put a large drop of oil onto the slide, and then rotate the 100X lens into the oil. When you are rotating the lenses, make sure that no other lens touches the oil! 4. When you are finished, you must clean the oil off of both the lens and the slide, as well as any you might have spilled on the microscope. 5. You should never use anything but LENS PAPER to clean the lens. Lens paper is specifically designed not to scratch the lens, and not to leave cotton fibers on the lens. 14 (ie-if you cleaned the lens with a paper towel, you would surely ruin the lens by scratching it! If you used Kleenex or other tissues to clean the lens, you would surely leave cotton fibers on the lens, which would make it difficult to see through.) 6. When you are cleaning the oil off the lens, slide and microscope, you must use a solvent that the oil is soluble in. In this case we will be using HEXANE. 7. The best way to clean the lens is to put a few drops of hexane on a piece of lens paper, and then rub in in a circular motion over the bottom of the lens. When you are finished, you can use the same piece of lens paper to clean the slide and microscope. (Try not to use too much lens paper, it’s expensive.) PROTOCOL: Looking at bacteria using oil immersion. 1. Obtain a bacterial slide from the back of the lab. (Remember to put the slide back in the correct box! The names of the bacteria are listed on the lid.) 2. Write down the name of the bacteria you are looking at. (It has two names, a Genus name and a species name. ie- Bacillus subtilis.) 3. Put the slide in the microscope, and focus it using the 4X lens. (Do not use any oil at this point. You’re viewing the slide with low power mainly to determine where the bacteria are before moving to a higher power. Under low magnification, the bacteria will appear as a purple patch on the slide.) 4. View the slide again using the 10X and 40X lenses, but do not use any oil. Can you see anything? 5. Finally, rotate the 40X lens out of the way, so that the slide is half way between the 40X and 100X lenses, and then put a large drop of oil on the slide. 6. Looking from the side, carefully rotate the 100X lens into the oil drop, and then raise the slide as high as it will go without touching the lens. (Remember, in order for this to work, the lens must actually be IN the oil.) 7. Look down the scope, and focus the image by moving the stage downward. 8. Identification of bacilli vs. cocci: A ‘bacillus’ is a bacterium that is rod-shaped, and a ‘coccus’ is a bacterium that is round. Write down whether you see bacilli or cocci. 15 EXERCISE VI EXERCISE VI: Using the dissecting microscope. The dissecting microscope is sometimes also referred to as the stereoscope. Biologists generally put large objects under the dissecting microscope in order to have a closer look at them. The specimen does not have to be cut into slices, and light does not pass through the specimen. Note that most dissecting microscopes have a zoom lens, so that you can increase or decrease the magnification without having to change lenses. You simply rotate the barrel of the lens. Place a coin under your dissecting microscope and take a look at it. Zoom the lens in and out. Based on what you see, estimate what the resolution limit is for a dissecting microscope. CLEAN UP: 1. Put all microscopes back where you found them. 2. If the microscope had a plastic dust cover (most of them do), put the dust cover back over it as well. 3. If you used the lamp, put the lamp back under the bench as well. 4. Put all slides back in their CORRECT BOXES (the lids of the boxes are labeled). 5. Put the slides back in their boxes STRAIGHT. (Notice that if you put a slide in the box at an angle, you will not be able to fit them all back in the box. Make sure the slots are lined up correctly.) 16 Part C: To Be Handed In Next Session. (8 points) This section is to be completed by you, printed out, and handed in at the beginning of the next lab period. (Fill in the answers by typing. Do not use handwriting.) Lab 1: Lab Report Name: CC Student Number: Course ID Number: 1. In your opinion, what was the purpose of this laboratory exercise? 2. What was the magnification power of the ocular lens on your compound microscope? 3. Lenses on your compound microscope: List the four magnification powers on the four objective lenses on your scope. 4. Which of the objective lenses was the OIL IMMERSION lens? 5. Which of the objective lenses was the SHORTEST (smallest)? 6. Question: you are viewing an object with the 100X objective lens. What is the TOTAL magnification? 7. Question: when biologists view things through a microscope at the cellular level, they usually describe how big things are in terms of “microns.” What is the word “micron” an abbreviation for? 8. How many microns are there in a meter? 9. How many microns are there in a millimeter? 10. How many nanometers are in a micron? 11. What is the ‘Refractive Index’ of air vs. an oil? You can look up the refractive index of any oil you like. Just mention to me which one you choose. (It’s alright to look this up on Wikipedia, or any other internet source, as long as you ‘cite,’ or mention that this is where you got the information from.) 17 EXERCISE IA: Label the parts of this compound microscope. EXERCISE IB: Briefly describe the function of each of the following parts of the compound microscope. 1. 2. 3. 4. 5. 6. 7. Ocular lens: Objective lens: Condenser: Condenser focus knob: Coarse focus knob: Fine focus knob: Stage: 18 EXERCISE 1C: Briefly explain why it is critical to always focus a microscope by lowering, rather than raising the stage. EXERCISE 1D: Briefly explain what parfocal lenses are. (Hint: the answer is in Section B, if you read it carefully.) EXERCISE 2: Estimating the size of a plant cell. 1. List the diameter of the field of view when you are using A. The 4X objective lens (40X magnification, total) B. The 10X objective lens (100X total) C. The 40X objective lens (400X total) D. The 100X objective lens (1000X total) 2. When you viewed the plant cells at 400X(total), approximately how many cells did it take to span from one side of the field of view to the other? 3. Based on this estimate, and your knowledge of how big the field of view is at 400X, what is the average size of a plant cell. EXERCISE 3: Estimating the size of a human erythrocyte. Using the same method you used above, what was your estimate of the size of a human erythrocyte? EXERCISE 4: Estimating the resolution limit of the compound microscope. What would you estimate to be the resolution limit of the compound microscope at 400X magnification? EXERCISE 5: Oil Immersion Light Microscopy. 1. List the name of the bacterium that you looked at. 2. Was this bacterium a bacillus or a coccus? EXERCISE 6: Using the dissection microscope. What would you estimate to be the resolution limit of a dissection microscope at maximum zoom? 19 LABORATORY EXERCISE 2: Cells and Osmosis Laboratory Exercise 2: Cells and Osmosis You have learned what happens to cells when placed in a hypotonic, isotonic, or hypertonic solutions, as a result of cells having selectively permeable membranes. A cell placed in a hypotonic solution will gain mass, as water moves into the cell in an attempt to equalize the concentration of solutes on either side of the membrane. The opposite will happen to a cell placed in a hypertonic solution. A cell placed in an isotonic solution will neither gain nor lose mass. You will use this principle to determine the concentration of solute inside a chicken egg. A chicken egg is essentially a single cell, covered with a protective shell. The protective shell can be removed by soaking the egg in acetic acid (vinegar), thus leaving just the selectively permeable cell membrane. You and your lab partners will place six eggs in a 0%, 10%, 20%, 30% or 40% solution of sucrose, and weigh them at 15 minute intervals to see if they gain or lose mass. You will then determine which solution is closest to being isotonic with the interior of the egg by calculating which egg has gained or lost the least mass. Note that the solutes inside the egg are mainly salts and protein, while the solute outside the egg is sucrose; but this makes no difference. As far as the movement of water is concerned, it is the net concentration of solute that matters, not what the solute is. Part A: Pre-Lab Quiz Questions. (2.5 points) 1. What is the solute used in this experiment to generate an osmotic potential outside the eggs, and what type of macromolecule is it classified as? 2. What types of macromolecules do you think are generating the osmotic potential inside the eggs? Part B: Pre-Lab Exercise. Must be handed in at the BEGINNING of the lab (2.5 points) Lab 2 Pre-Lab Exercise Name: CC Student Number: Course ID Number: Expressing Experimental Results in the Form of Graphs: The purpose of this pre-exercise is to give you some experience creating and interpreting graphs. It is not meant to be an exhaustive discussion of how and when to use different types of graphs, 20 and you will learn more about graphing in other courses. introduction to the principles of graphic literacy. This is only meant to be an Like other scientists, biologists often use graphs to illustrate the results of their experiments. Graphs are a very fast and effective way of proving a scientific point. A picture is worth a thousand words! While there is no standardized method of using graphs to show results (iedifferent scientific journals may impose different rules on the kinds of graphs biologists can use to show their data), the main goal of a graph is to show differences. For example, a biologist will do an experiment where they give two different treatments to two identical (or nearly identical) groups of subjects, and then hope for two different outcomes. They will then plot the results on a graph in a way that best highlights any differences in the outcomes. If the purpose of drawing a graph is to make it easy for your readers to see any differences in your results two general principles are worth remembering. 1. It is best to plot the data from the two groups on the same graph, rather than on two different graphs. Plotting two different sets of data on the same graph means you will have to use different symbols for each data set, and you’ll have to include a ‘legend’ which tells the reader which set is which. 2. If you are going to plot two data sets on the same graph, it is best to convert them to some common unit, if possible. We’ll discuss what this means below. Converting Data to a Common, Comparable Unit: Suppose you were trying to prove that a new type plant fertilizer makes both apple trees and flowers grow faster than the standard fertilizer. Looking at the flowers first, you would do the experiment by taking two groups of flowers, putting the standard fertilizer in the soil of one group, the new fertilizer in the soil of the other group, and then measuring the heights of the two groups every week. The group of flowers that you added the standard fertilizer to is called the control group, and the group that you treated with the new fertilizer is called the test group, or experimental group. Suppose you did this experiment, and got the following results: A) Week 0 1 2 3 TABLE 1: Effect of Fertilizer on Flower Growth B) Control C) Test D) Difference E) Difference (cm) (cm) (cm) (%) 1 1 0 100 10 20 10 200 20 60 40 300 30 180 150 600 In this experiment you planted 100 seeds in each of two different plots of soil. You then add the two types of fertilizer when each group is 1cm tall, and measure the growth of the flowers every week. The average height of each group is recorded in this table. Column A shows the time in weeks, and should be put on the X-axis of any graphs, because it is what’s called the ‘independent variable.’ (The passage of time is constant, and has nothing to do with what kind of fertilizer you use. Thus, it is independent of the experimental conditions.) Columns B and C 21 are the average heights of the 100 plants in each group. Column D shows the difference between the test group and the control group, expressed in centimeters. Column D was derived by subtracting the average height of the control group from the average height of the test group at each of the time points. (ie-20cm – 10cm = 10cm, for the first week.) Column E shows the difference between the test group and the control group expressed as a percentage. Column E was derived by dividing the height of the test group by the height of the control group, and then multiplying by 100%. (ie- 20cm/10cm X 100% = 200%, for the first week.) EXERCISE 1: Plot a graph with the data from Table 1, using Column A on the X-axis, and Columns B and C on the Y-axis. Call it Graph 1. Because you will be plotting two lines on the same graph (control group and test group) you will have to use two different symbols for each of the data points, and include a ‘legend’ explaining which is which. The following is an example of a two line graph with a legend. (Note that this graph has two Y-axes. We’ll discuss when and why to use a double Y-axis later.) You may either use a piece of graph paper, or a computer program (like Microsoft Exel) to draw your graph. The same applies to all the other graphs you will plot for his assignment. (The purpose of this assignment is to learn how to best illustrate data using different types of graphs, and not to learn how to use graphing programs on the computer. Therefore, I will not penalize you for drawing the graphs by hand. However, it is to your advantage to learn how to use graphing programs on the computer. So, if you do not know how to use one, and you have the time to learn, I would advise that you do so. Microsoft Excel is quite simple to use, and you could probably learn how to use it to plot graphs from one of your friends in a few minutes.) EXERCISE 2: Plot Columns A and D from Table 1 in a graph. Call it Graph 2 EXERCISE 3: Plot Columns A and E from Table 1 in a graph. Call it Graph 3 EXERCISE 4: 22 Answer the following question: which of these three graphs do you think best shows the increase in height caused by the new fertilizer, and why? EXERCISE 5: Calculate another column for Table 1, by subtracting 100% from each of the values in Column E. Column E gives you the size of the test group relative to the control group. By subtracting the size of the control group (as a percentage) from the test group, you will see how much bigger the test group is than the control group ( as a percentage). In other words, the new data set will tell you how many percent bigger the test plants are than the control plants at each time point. Plot these values on a graph. Call it Graph 4. You now do a similar experiment with apple trees, and get the following results: A)Week 0 1 2 3 TABLE 2: Effect of Fertilizer on Apple Tree Growth B)Control C)Test D)Difference E)Difference (cm) (cm) (cm) (%) 200 200 400 800 600 1200 800 1600 F)Net Diff. (%) In this case, the fertilizers were added to the apple trees when they were already 200cm tall. Note that I have not calculated the last three columns for you. You must do this, including Column F, which is again calculated by subtracting 100% from each of the values in Column E. EXERCISE 6: Plot three different graphs using the data from Table 2. The three graphs should use Columns D, E and F, and be called Graphs 5, 6, and 7, respectively. EXERCISE 7: Answer the following question: Of graphs 5, 6, and 7, which do you think best highlights how much better the new fertilizer makes apples trees grow than the standard fertilizer, and why? EXERCISE 8: (This exercise is more challenging!) Now, compare the effect of the new fertilizer on the growth of apple trees vs. flowers. The purpose of this graph is to show which of these two types of plants is more strongly effected by the new type of fertilizer. Plot a graph with two lines on it, one for flowers and one for apple trees. Use a legend to indicate which is which. You must use WEEKS on the X-axis, but you may choose any two columns from Tables 1 and 2 for the growth data. Chose the two columns that you think will show the greatest difference. (If necessary, you can use two Y-axes.) Call it Graph 8. 23 A) Week 0 1 2 3 TABLE 3: Effect of Fertilizer on Number of Apples per Tree. B) Control (apples/tree) C) Test (apples/tree) 0 0 20 10 30 20 35 25 Now, suppose that the reason you wanted to test this fertilizer on flowers was because you wanted to grow the flowers as fast as possible to send them to market. On the other hand, your purpose in testing the fertilizer on apple trees was to hopefully make the trees grow bigger, and produce more apples to send to market. You’ve already proven that the fertilizer makes both the flowers and trees grow faster, and to a greater height; but you have not proven that the taller apple trees will produce more apples. Data on the number of apples per tree is presented in Table 3. The data in Table 3 are from the same trees as in Table 2. (You simply counted the number of apples on the trees at the same time you were measuring their height and growth rate.) EXERCISE 9: Using data from Tables 2 and 3, plot a graph showing both growth rate AND number of apples per tree on the same graph. (This is a perfect example of a situation in which you’d need to use two different Y-axes, because you are attempting to measure two different ‘dependent variables’ simultaneously. Time (in weeks) is still the independent variable, and tree height and number of apples are the two dependent variables.) Plot a graph comparing both the difference in height, and the difference in apple production for the two groups using any columns from Tables 2 and 3 you like. In this case you’ll have to use four lines and four sets of symbols. Call it Graph 9. EXERCISE 10: Answer the following questions: Would you recommend using the new fertilizer to grow flowers? Why or why not? Would you recommend using the new fertilizer to grow apples? Why or why not? Minutes 0 30 60 Table 4: Weight of Eggs in Sucrose Solutions 0% sucrose 20% sucrose 20g 22g 24g 23g 28g 24g 40% sucrose 26g 24g 22g The data recorded in Table 4 are similar to what you might get with the experiment you are about to do. In this experiment you weigh three eggs before putting them in sucrose solutions. You then put them into sucrose solutions as shown, and re-weigh them every 30 minutes. EXERCISE 11: Using the data from Table 4, plot a graph with three lines on it, showing the weight of the eggs vs. minutes without any treatment of the data. Call it Graph 10. Then convert the weight of each egg to a percentage, relative to what each egg weighed at the beginning. (For example, the egg in the 0% sucrose solution weighs 20g at the beginning, and 24g after 30 minutes. Thus, 24/20X100% = 120%. After 30 minutes, the egg weighs 120% of what it weighed at the 24 beginning.) Plot another graph which shows the gain or loss of egg mass as a percentage of what each egg weighed at the beginning. Call it Graph 11. Finally, subtract 100% from each of these values, to give you just the CHANGE in mass (as a percentage) over time. (For example, the 0% sucrose egg weighs 120% of what it weighed in the beginning. If we subtract 100% from this number, we see that the egg gained 20% mass after 30 minutes. Note also that the relative mass of the egg was 0 at the beginning.) Plot a final graph showing just the percentage increase or decrease in egg mass (as a percentage) over time. Call it Graph 12. EXERCISE 12: Answer the following question: which of Graphs 10 through 12 do you think illustrates the change in egg mass over time most clearly? Part B: Lab Protocols. Determining the change in mass of chicken eggs soaked in various concentrations of sucrose. (Warning: handle the eggs carefully. They are very fragile when the shell has been removed.) The four people in your lab group must do this experiment together. Make sure everybody has a chance to weigh a few of the eggs, and get some experience using the top loading balance. a) Obtain six chicken eggs from the bucket at the back of the room. The shells on these eggs have been slowly dissolved away using acetic acid. The membrane that remains is called the shell membrane, and it is differentially permeable. Assume that each egg has approximately the same concentration of solute inside the shell membrane. Based on the rate of osmosis that you observe in these eggs, you will try and determine what the concentration of solute is inside the eggs. b) Obtain six Styrofoam bowls (one for each egg) and label them 0%, 10%, 20%, 30% and 40%. (Note: the previous lab might have already labelled the bowls. If they did, you don’t have to re-label them.) c) Weigh each egg separately to the nearest 0.1g and record the mass in Table 1 at “Time 0”. When weighing an egg, carefully wipe off any excess solution from the outside of the membrane so that you are weighing the contents of the egg only. d) How to weigh the eggs: You will be using a type of weighing balance called a ‘TopLoading Balance.’ The balance is supplied with something called a ‘weighing boat.’ The weighing boat has a mass of its own, which must be subtracted before you put an egg in it. To subtract the weight of the boat, put the boat on the balance while the balance is turned on. (Notice the weight of the boat.) Then, push the button marked ‘TARE’ to subtract the weight of anything that is already on the balance. (Notice that the weight went back to zero.) Now you can place an egg into the boat, and record the 25 weight of just the egg. You may have to wait a few seconds until the balance stabilizes, and the number on the screen stops fluctuating. e) Place each egg into a cup so that there are six cups labeled with the six different concentrations of the solute sucrose - 0% (water), 10% sucrose, 20% sucrose, 30% sucrose, and 40% sucrose. f) Pour enough of the respective solutions into the cups so that the eggs are completely submerged. g) Every 15 minutes, remove the eggs from the beakers and weigh each egg separately. Again, remember to wipe off the egg before measuring. Put the egg back in the solution once you’ve measured the weight. h) Record your data in Table 1. Table 1. The mass of eggs subjected to varying concentrations of sucrose. SUCROSE CONCENTRATION (%) TIME (MIN) 0 10 20 30 40 0 15 30 45 60 75 Clean Up: 1. Put the eggs into the green discard bucket. 2. Wash out the Styrofoam bowls and weighing boat and put them back in a stack on your bench, as you found them. 3. Carefully give the top of the Top-Loading balance a wipe with a wet paper towel. 4. Use a wet paper towel to wash off the top of your lab bench. (Sucrose is sticky!) 5. Put all paper towels in the regular garbage. 26 Part C: To Be Handed In Next Session. (5 points) Lab 2: Lab Report Name: CC Student Number: Course ID Number: 1. Plot a graph showing any increases or decreases in mass of the eggs when soaked in different sucrose concentrations. (You may use either graph paper or a computer program to plot the graphs. Don’t forget to include a legend. Use the method of graphing that will best highlight any differences!) 2. Based on your results, which concentration of sucrose is closest to the osmotic concentration inside the eggs? Explain your reasons for thinking so. 27 LABORATORY EXERCISE 3: Characteristics of Enzymes Laboratory Exercise 3: Characteristics of Enzymes In this lab you will use a biochemical reagent called Lugol’s Solution to estimate the optimum pH for human amylase (contained in human saliva) and plant amylase. Part A: Pre-Lab Quiz Questions (2.5points) 1. What is Lugol’s Iodine, and what exactly does it measure? 2. Which enzyme are we looking at in this lab, what is its SUBSTRATE, and (in the case of the human enzyme) what organs produce it? 3. If you add Lugol’s Iodine to a solution and it turns dark brown does it mean that there is a large amount of starch present in the solution, or a small amount? 4. If you add Lugol’s Iodine to a solution and it turns light brown, or light brownish yellow, does it mean that there is a large amount of starch present in the solution, or a small amount? Part B: Pre-Lab Exercise. This section to be handed in at the start of class (2.5 points) Lab 3 Pre-Lab Exercise Name: CC Student Number: Course ID Number: Optimum Conditions for Enzyme Activity: As you have learned in lecture, enzymes are proteins that catalyze chemical reactions. Most enzymes have optimum conditions under which they work best, including an optimum temperature, and optimum pH and an optimum substrate concentration. The following table contains a typical set of results that you might get if you did three separate experiments to determine the optimum pH, temperature and substrate concentration for an enzyme. In this case, the enzyme is an amylase enzyme that converts starch to glucose. This amylase enzyme is produced by an acidophilic bacteria called Acetobacter aceti. Acetobacter aceti is the bacterium that converts sugars into acetic acid (often used in the food industry to make vinegar). Use the data in Table 1 to find the optimum conditions for this amylase enzyme. Find a way to put all three sets of data onto the same graph. Obviously, you will have to include a legend. You may also wish to use a double X-axis or a double Y-axis. Note that, in order to do an experiment where you are measuring the effects of three different variables (pH, temperature and substrate concentration), you have to hold two of the three variables constant while varying the third. For example, when these researchers measured the effects of pH, they had to vary the pH while holding the temperature and substrate concentration constant. The purpose of this experiment was to determine the optimum conditions for this 28 enzyme, and the researchers didn’t know these conditions beforehand. Therefore, when they were measuring one condition, they picked an arbitrary value to hold the other two conditions at. In this experiment the temperature was held at 20°C and the substrate concentration was held at 10mM while pH was being measured; the pH was held at 3.0 and the substrate concentration was held at 10mM while the temperature was being measured; and the pH was held at 3.0 and the temperature was held at 20°C while the substrate concentration was being varied. 29 Table 1: Amylase Enzyme Efficiency as a Function of pH, Temperature, and Substrate Concentration pH Enzyme Temp. Enzyme Substrate Enzyme Efficiency (°C) Efficiency Concentration Efficiency (%) (%) (mM) (%) 0 0 0 0 0 0 0.5 3 5 2 5 20 1.0 12 10 2 10 28 1.5 31 15 2 15 33 2.0 40 20 2 20 36 2.5 31 25 5 25 38 3.0 12 30 15 30 40 3.5 3 35 25 35 41 4.0 0 40 15 40 42 4.5 0 45 5 45 42 5.0 0 50 2 50 42 5.5 0 55 2 55 42 6.0 0 60 2 60 42 6.5 0 65 2 65 42 7.0 0 70 2 70 42 Answer the following questions: 1. What is an ‘acidophilic bacterium?’ 2. Do you think the optimum pH for this enzyme is consistent with the fact that the bacterium that produces it is an acidophile? 3. What is the ‘substrate’ for an amylase enzyme, and what is the ‘product’ of the reaction? 4. Why do you think the enzyme efficiency, as measured in this experiment’ never reaches 100%? (Hint: the answer is in the last paragraph above the table, where the experimental conditions are discussed.) 5. Notice that the pH and temperature data form two ‘peaks,’ while the substrate data forms a ‘plateau.’ The plateau is reached when you give the enzyme the optimum amount of substrate to work with, but neither increases nor decreases when you give it any more substrate. Can you explain why this would be the case? (Note: being able to explain this clearly is probably the most difficult part of this assignment.) 30 Part B: Lab Protocols. In this lab you will estimate the optimum pH of human and plant amylases. We will provide you with the plant amylase, and you will provide the human amylase in the form of saliva. One member of your lab group will have to put 20mL of water in their mouth, swish it around for two minutes, and then spit it back into a cup. (Sorry, we’re aware that this is disgusting.) Preferably, this should be done by somebody who has not eaten recently. You will take six test tubes containing a starch solution, and add buffers to the tubes so that they will be held at a pH of 3, 4, 5, 6, 7, or 8. You will then add either human or plant amylase to these tubes, incubate them in a 37°C water bath, and remove aliquots every three minutes to measure the degree of hydrolysis. You will estimate the degree of hydrolysis by adding three drops of a biochemical reagent called Lugol’s Iodine (a mixture of iodine and potassium iodide). In the presence of starch, Lugol’s Iodine stains the starch black. In the absence of starch, Lugol’s Iodine appears light brown or yellow. Thus, you can estimate the degree of hydrolysis by recording a black colour as +++, a yellow colour as 0 (no reaction), or the varying degrees of brown as ++ or +; giving you a scale of +++, ++, +, and 0. The more starch hydrolysis that has occurred, the lighter the solution will be. You will measure the degree of hydrolysis in something called a depression plate, where you add three drops of Lugol’s Iodine to each well in the plate BEFORE adding your sample to it. Adding Lugol’s Iodine to the enzyme/starch mixture will stop the reaction. Therefore, you cannot simply add the Lugol’s Iodine to the test tubes at the beginning, and then take aliquots out every three minutes to estimate the colour, because the Lugol’s solution would stop the reaction as soon as it was added. The best way to do the experiment is to first set up and label your plates (as seen in Figure 1), and add three drops of Lugol’s Iodine to each well. Then, when you remove your aliquots from the test tubes, you simply suck up 1 mL of the reaction mixture, and drop it into a well which already contains the Lugol’s. (Question: What do you think would be the result if you took your aliquots of enzyme/substrate and put them into the wells of the plate every three minutes, and then added the Lugol’s Iodine to each well at the END of the experiment, rather than adding Lugol’s to each well at the BEGINNING?) Do the experiment in the following sequence: Do the human amylase experiment first. Obtain the amylase sample (saliva), set up and label the test tubes and the depression plate, and do the experiment. Then wash out and dry the depression plates, obtain six more tubes, and repeat the experiment for the plant amylase. Make sure everybody in the group gets to take some of the time points. Protocol 1: Determining the Optimum pH of Human Salivary Amylases. 1. Take 6 test tubes and label them 3 through 8 with a permanent marker. (Note: the previous lab group probably already labelled the test tubes, in which case you should simply look for six tubes that are already marked.) 2. Put 2mL of starch solution into each test tube using a plastic squeeze bulb marked with a 1mL gredation. 31 3. Add 1mL of the appropriate buffer solution to each labeled test tube (i.e. pH 3 goes in tube labeled 3 etc.). Use the squeeze droppers contained in the lids. DO NOT MIX THEM UP! Note: 1ml is approximately 20 drops. 4. Collect plastic squeeze pipettes, place one in each tube, and mix the contents. Leave the pipettes in their respective tubes. 5. Set up and label 2 depression spot plates as illustrated in Figure 1, and then add 3 drops of Lugol’s Iodine to each well. (Note: the previous lab group might have already labelled the plates, in which case you don’t have to do it again.) 6. Place your tubes in a water bath that has been pre-set to 37ºC. (You do this before adding the amylase in order to give the solution a chance to heat up to 37°C before you add the enzyme.) 7. Using the sterile cup provided, add approximately 20mL of tap water to the cup. 8. Pour all the water into your mouth and swish it around your mouth for at least 2 minutes. When time is up, spit it back into your cup. 9. Use a squeeze pipette to draw 2mL of your saliva sample and add it to each test tube. Keep the test tubes in the water bath for the rest of the experiment. 10. Set your timer for 3 minutes. 11. Using the pipettes that you placed in each tube, quickly mix the contents together by swishing them around. Immediately add 3 drops from each tube to the appropriate well (add 3 drops of the mixture in test tube #3, to well 3-1, then 3 drops of the mixture in test tube #4 to well 4-1, etc). This is time 0. 12. Start your timer. 13. Repeat this process at 3 minute intervals into the appropriate well until 9 minutes has elapsed and every well is used up. pH 3 will only go in column 3, likewise pH 4 in column 4 etc. 14. Dispose of your plastic cup into the garbage bins and wash the squeeze bulbs as well as all glassware and plates thoroughly. 15. Dry the depression plates for use in the second experiment. You can leave the wet test tubes and squeeze bulbs to dry by the sink. Obtain new, dry test tubes and squeeze bulbs for the next part of the experiment. 32 PROTOCOL 2: Determining the Optimum pH of a Plant Amylase. 1. Repeat the same protocol as above, except this time you add 2 mL of plant amylase, instead of saliva solution. (You can use a clean, dry squeeze pipette to add the plant amylase). pH 3 4 5 6 Part C: To Be Handed In Next Session. (5 points) Name: 0 7 8 Lab 3: Lab Report 3 CC Student Number: Time Course ID Number: (minutes) 6 9 FIGURE 1: Depression Plate Set Up Clean Up: 1. Wash and dry all of the depression plates, and return them to the back bench where you found them. 2. Wash all of the test tubes and squeeze bulbs. 3. Tidy up your bench, return all the reagents to the basket where you found them. 4. Give your bench top a wipe with a damp paper towel. 33 Part C: To Be Handed In Next Session. (5 points) Lab 3: Lab Report Name: CC Student Number: Course ID Number: 1. Draw a graph showing your rough estimate of starch hydrolysis from human and plant amylases. (Remember that +++ means a LOW level of hydrolysis, and + means a HIGH level of hydrolysis.) 2. From your results, what would you estimate the optimum pH to be for human and plan amylases? 3. Is the optimum pH the same for human and plant amylases? If not, can you suggest a reason why it might not be? 34 LABORATORY EXERCISE 4: Photosynthetic Plant Pigments Laboratory Exercise 4: Photosynthetic Plant Pigments In this lab you will use a technique called Paper Chromatography to separate a mixture of three different types of photosynthetic plant pigments. Part A: Pre-Lab Quiz Questions. (5 points) Lab 4 Pre-Lab Quiz Questions The word ‘Chromatography’ refers to one of several related methods that biochemists use to separate mixtures of biological molecules into components for individual analysis. Paper Chromatography (also known as Thin Layer Chromatography, and abbreviated TLC) was the first method developed to do this. TLC was later modified into two more advanced methods, which are abbreviated FPLC and HPLC. FPLC and HPLC are routinely used by biologists and biochemists today, and you should be familiar with what they are. Questions: 1. What does FPLC stand for? 2. What does HPLC stand for? 3. Which photosynthetic pigment do you expect to find at the BOTTOM of your chromatogram? (Answer in the protocols) 4. Which photosynthetic pigment do you expect to find at the TOP of your chromatogram? (Answer in the protocols) 5. In this type of chromatography, which is the “solid phase?” 6. In this type of chromatography, which is the “liquid phase?” 7. Is the solvent that will be used in this lab polar or non-polar? 35 Part B: Lab Protocols. Chromatography is a biochemical technique used for the separation of various compounds from solution. Separation of compounds by this technique is based on differences in their solubility in two phases: stationary and mobile. The stationary liquid phase, usually a water solution, is a polar solvent whereas the mobile liquid phase is a more non-polar organic solvent. In paper chromatography the stationary phase is created by hydrogen bonding between the water and the cellulose fibres of the paper. The mobile phase begins at one end of the stationary phase and moves toward the other, percolating between the fibres of paper by capillary action. Within the mobile phase, the solutes are separated in a distinct pattern characteristic of the particular combination of support medium and developing solvent used. Under identical sets of conditions, the distance a given solute travels from the origin is a constant fraction of the distance the solvent travels. This decimal fraction is the Rf (Relative to the front) value for that specific solute. This value can be used to help identify a particular solute on a given chromatogram. The Rf can be calculated for this example as follows: Rf = Distance to solute band = 8 cm = 0.8 Distance to solvent front 10 cm Some factors which might affect Rf values are temperature, the extent to which the environment in which the chromatogram is run has been saturated by the developing solvent, variations in solvent composition, and variations in stationary phase composition. The extent to which any solute will follow the solvent front is dependent upon two things: 1) its solubility in the developing solvent, and 2) adsorption (the tendency for the solute to bond to the stationary phase). 36 Both solubility and adsorption are related to the polarity of a compound. Hydroxyl groups on the cellulose molecules of the chromatography paper give the stationary phase an affinity for polar solvents and solutes. For this reason polar molecules tend to be adsorbed while less polar molecules are not. Organic solvents, on the other hand, are more non-polar in nature (dissolving and carrying non-polar solutes). Hence, a polar solute would tend to adsorb to the stationary phase and not move with the solvent. A non-polar solute would dissolve readily in the organic solvent, would not tend to be adsorbed, and would thus follow the solvent front. Many compounds have both polar and non-polar groups. Such a molecule in a non-polar solution would be slowed by temporary attachment to the polar stationary phase. For example, a molecule of type X may spend half its time attached to the stationary phase. Even though all type X molecules would not be attached at the same time, the net result would be a band of molecules that travels half the distance from the origin to the solvent front. An Rf for this particular molecule under these conditions would be Rf = 0.5. The longer a chromatogram runs, the more any given band tends to widen. This is due to variations in path length caused by molecules taking different routes between the cellulose fibres. The most common plant pigments are the carotenes, xanthophylls, and chlorophylls. In paper chromatography beta-carotene, the most abundant of the carotenes, occurs almost at the solvent front. It is very soluble in the solvent and has no atoms that would form hydrogen bonds with the cellulose of the paper. Xanthophyll is different from beta-carotene because it contains oxygen. It is slowed down by hydrogen bonding with the cellulose, and is less soluble in the solvent, so it occurs lower down on the paper strip. The chlorophylls are the lowest pigments on the chromatogram because they contain both oxygen and nitrogen and are held more tightly to the cellulose. The primary photosynthetic pigment in plants is chlorophyll a. Chlorophyll b, carotenes, and xanthophylls act as accessory pigments that capture light energy and transfer it to chlorophyll a. The carotenes and xanthophylls also protect the photosynthetic apparatus of the plant from being damaged by bright sunlight. PROTOCOL: 1. In a mortar place 1 or 2 leaves/pieces from one of the plants provided. Add about 2 mL of extracting solvent and a pinch of sand. Grind well with a pestle for one minute to extract the pigments. 2. Attach a strip of chromatography paper to a cork that fits the test tube provided, so that the tip of the paper just reaches the bottom (see figure below). 37 3. Using a capillary tube make a spot of pigment extract on the paper strip 1 cm from the end opposite the cork. Dry the spot after each application to keep it from spreading. Repeat this until the spot is very dark green or black. Try to keep the spot as small as possible by placing only a small amount of pigment extract on the paper at one time. 4. Suspend the paper strip and the cork so that the paper extends into the solvent, but make sure the pigment spot sits above the level of the solvent. Be sure the strip does not touch the sides of the cylinder or that solvent does not splash the paper. 5. Place the test tube in the rack, and your instructor will take it to the fume hood in the chemistry lab next door, and place the solvent in it. 6. Let the chromatogram run for 20-30 minutes or until the solvent reaches 3/4 of the way to the top of the strip 7. Remove the strips, mark the furthest distance travelled by the developing solvent (the solvent front), then allow the chromatogram to dry. (Note: mark solvent front as soon as you take the chromatogram out of the tube. The solvent will evaporate quickly, and if you don’t mark it right away you won’t know where it was.) 8. Tape the chromatogram to a separate sheet of clear white paper, and do the following: a) Number each figure and provide an appropriate title. b) Name the plant material used and the solvent used. c) Label the solvent front. d) Observe the various bands visible under natural light; outlining each with a pencil. e) Observe the various bands visible under U.V. light; and note how these differ from the bands visible in 'e' above. f) Label the various pigments to the best of your ability, using the information provided below. g) Calculate the Rf values for each pigment. 38 Note: The probable sequence for chromatographic pigments on your chromatogram sample would be: · · · · · solvent front carotenes (yellow) one or more bands of xanthophylls (yellow) chlorophyll a (blue-green) chlorophyll b (olive green) Approximate Rf results would be: Rf carotene = 0.95 - 0.99 (very faint yellow line) Rf xanthophyll = 0.4 (yellow) Rf chlorophyll a = 0.2 (bright green to blue green) Rf chlorophyll b = 0.1 (yellow green to olive green) These values are approximate, and yours may vary. 39 Part C: To Be Handed In Next Session. (5 points) Lab 4: Lab Report Name: CC Student Number: Course ID Number: 1. Include your chromatogram in your lab report. (Either staple of tape the chromatogram to one of the internal pages.) 2. Include your raw measurements, as well as your calculated Rf values for each pigment. 3. Answer the following questions: Q1. How does your chromatogram differ from one that another group did with a different plant? How are they similar? Q2. Which pigments fluoresce? What colour does each of the fluorescent pigments appear under UV light? Q3. How can you explain the presence of colours on the chromatogram that are absent in the original leaf? Q4. Explain how you would account for the differential rates of migration of the various pigments using specific examples. What is the principle behind the separation? 40 LABORATORY EXERCISE 5: Plant Anatomy Laboratory Exercise 5: Plant Anatomy In this lab you will examine several types of plant tissue under the microscope. Part A: Pre-Lab Quiz Questions. (5 points) Lab 5 Pre-Lab Quiz Questions In your own words, define each of the following terms. 1. Apical bud 2. Axillary bud 3. Collenchyma 4. Cotyledon 5. Ground tissue 6. Meristem 7. Parenchyma 8. Petiole 9. Phloem 10. Sclerenchyma 11. Sclerid 12. Xylem 41 Part B: Lab Protocols. 1) Primary growth and development Plants produce new cells throughout their lifetime as a result of cell division in meristems. Tissues produced by apical meristems are called primary tissues, and this growth is called primary growth. Primary growth occurs along the plant axis at the shoot and root tip. Certain meristem cells divide in such a way that one cell product becomes a new plant body cell and the other remains in the meristem to continue dividing. Beyond the zone of active cell division, new cells become enlarged and specialized for specific functions (protection, storage, water transport, etc). The shoot apical meristem: Observe the demonstration slide of a longitudinal section through a terminal bud (shoot apex) of the eudicot Coleus. Use low power to observe the organization of the primary meristems and tissues and medium/high power if you wish to examine specific cells or locations in the shoot. Below is a diagram of the Coleus shoot apex. Label the following structures/regions: A. apical meristem B. axillary bud C. immature leaf 42 The root apical meristem: Observe the slide of a longitudinal section through a growing root of the monocot Allium (onion). Use low power to observe the organization of the primary meristems and tissues and medium power to examine specific cells and locations at the shoot and medium/high power if you wish to examine specific cells or locations in the shoot. Draw a generalized diagram of the Allium root tip labeling the following structures/areas: A. Zone of cell division B. Zone of Elongation C. Zone of maturation D. Root cap E. Root hairs 2) The structure of stems A stem is usually the main stalk, or axis, of a plant and is the only organ that produces buds and leaves. Stems support leaves and conduct water and inorganic substances from the roots to the leaves and carbohydrate products of photosynthesis from the leaves to the roots. You will view prepared slides of monocot and eudicot stems to gain an appreciation and understanding of the inherent differences in their construction and organization of the primary tissues. In addition, you will make your own slide of a living eudicot stem to observe these tissues. Monocot versus eudicot stems: 1. Observe the demonstration slide of cross sections through a monocot and a eudicot stem. You will have to move around the slide and view each at one time. Use low power to observe the organization of the primary tissues and medium power to examine specific tissues and cell types. 2. Obtain a stem of Coleus and make your own cross section through a young portion. Be sure to observe a demonstration of how to make a thin cross section if you are unsure. Use methylene blue (a stain) to assist in differentiating between structures. Draw a generalized diagram of the Coleus stem cross section showing the organization of the primary tissue systems. 43 3) The structure of roots Roots and stems often appear to be similar, except that roots grow in the soil and stems above the ground. Though composed of the same three primary tissues as stems, the organization of these tissues is different in roots and consequently the function of the root is inherently different from that of the stem. Monocot versus eudicot roots: 1. Observe the demonstration slide of cross sections through a monocot root. Use low power to observe the organization of the primary tissues and medium power to examine specific tissues and cell types. 2. Observe the slide of cross sections through the Ranunculus root (eudicot). Use low power to observe the organization of the primary tissues and medium power to examine specific tissues or cell types 4) The structure of leaves Leaves are organs especially adapted for photosynthesis. The thin blade portion provides a very large surface area for the absorption of light and the uptake of carbon dioxide through stomata. The leaf is basically a layer of parenchyma cells (the mesophyll) between two layers of epidermis. The loose arrangement of parenchyma cells within the leaf allows for an extensive surface area for the rapid exchange of gases. Specialized epidermal cells called guard cells allow the exchange of gases and the evaporation of water at the leaf surface. You will examine the structure of monocot and eudicot leaves in cross section and observe the stomata on the leaf epidermis. 1. Observe the demonstration slide of a cross section through a leaf. Use low power to observe the organization of the primary tissues and medium power to examine specific tissues and cell types. Describe the basic construction of the leaf, noting which slide you viewed, the organization of the three primary tissues and sizes and shapes of the cell types. 2. Observe the demonstration slide of a whole mount of Tradescantia leaf epidermis. Notice the many guard cells that populate the epidermis. 5) Vascular Tissue Xylem and phloem comprise the two primary components of vascular tissue. Xylem contains specialized cells called tracheids and vessels specialized for long-distance transport 44 of water. Phloem, on the other hand, is specialized for translocation of sugars through cells called sieve-tube members with the assistance of smaller, nucleated companion cells. In the following exercises you will examine monocot and eudicot stem, root, and leaf cross sections and familiarize yourself with the structure of xylem and phloem cells. 1. Observe the slides of roots, stems, and leaves from the previous exercises and look for xylem and phloem. Try and differentiate between vessels and tracheids in the xylem and between sieve-tube members and companion cells in the phloem. Draw a diagram of a vascular bundle from one of the slides available in the lab and label the xylem and the phloem. Include the name of the slide that you viewed. 6) Collenchyma Collenchyma cells have an unevenly thickened primary cell wall. These cells function in support of growing stems and leaves and are alive at functional maturity, so that the cell grows along with the plant while supporting it. 1. Prepare your own slide of collenchyma cells by making a cross section through the petiole of celery (the ‘stalk’ that you eat). Use the stain provided to assist in differentiating different tissues and cell types of your slide. Draw a diagram of your cross section through the celery petiole, showing the locations of collenchyma cells. Draw some highly magnified collenchyma cells 7) Sclerenchyma Sclerenchyma cells have an evenly thickened secondary cell wall. These cells function in support of growing stems and leaves and unlike collenchyma are usually dead at functional maturity. Xylem vessels and tracheids are types of sclerenchyma cells as are the fibres you viewed in the previous section. Sclerids are other type of sclerenchyma cell that you will view. Prepare a slide of the fruit tissue of a pear. Do this by scraping a SMALL amount on a slide and observing under the microscope . 45 The sclerenchyma cells in the pear are called sclerids and they are what make the pear “gritty” when it is eaten. Scelrids do not function in water transport like xylem, which is why they can afford to be so irregular in shape. Draw some sclerids. 46 Part C: To Be Handed In Next Session. (5 points) Lab 5: Lab Report Name: CC Student Number: Course ID Number: 1. On the diagram below, label the following (by hand, if you like): A. Apical meristem B. Axillary bud C. Immature leaf 2. Sketch a sclerid. 3. Answer the following questions: A. What is the function of the Root Cap of a root? B. What is a Root Cap made of? C. List one main structural difference between monocot stems and eudicot stems. D. List one main structural difference between monocot leaves and eudicot leaves. E. List one main structural difference between monocot seeds and eudicot seeds. (Hint: this difference was used to derive the names for monocots and eudicots.) 47 LABORATORY EXERCISE 6: Digestive Systems Laboratory Exercise 6: Digestive Systems In this lab you will examine the digestive systems of rats, frogs, worms and other animals for the purpose of understanding how digestive systems work. The structures of the digestive systems of various organisms may differ, but the basic principles of how they work remain the same. In all cases, food is broken up into small fragments in order that digestive enzymes may have a larger surface area to work with. The enzymes then proceed to break down the food into its component macromolecules, and these macromolecules are absorbed by the organism as the food transits through a long tube composed of epithelial tissue. Part A: Pre-Lab Quiz Questions. (5 points) Lab 6 Pre-Lab Quiz Questions Part A. The Alimentary Canal: In your own words, describe the functions of each of the following components of the human digestive system. (The same functions apply to all mammals, including the rat.) 1. Teeth 2. Tongue 3. Salivary glands 4. Esophagus 5. Esophageal sphincter 6. Cardiac sphincter 7. Stomach 8. Pancreas 9. Liver 10. Gall bladder 11. Pyloric sphincter 12. Duodenum 13. Small intestine 14. Large intestine Part B. ODONTOLOGY: The following are three different types of teeth that mammals often have. In your own words, describe the function of each. 1. Incisors 2. Canines 3. Molars 48 Part B: Lab Protocols. The digestive systems of animals vary depending on the kind of food the animal eats. Accordingly, animals may be grouped as carnivores (meat eaters), herbivores (plant eaters), omnivores (both meat and plant eaters), or as filter feeders (eaters of small and microscopic animals) or fluid feeders (eating by sucking fluids from plant and animal tissues). In this exercise, as you observe the digestive system of each animal, try to determine how the structure of the digestive system is adapted to digest its food. Each group should obtain a rat, a frog and a worm from the common buckets. Also, each group should obtain a large plastic bag, and write all the group members’ names on it, as well as your lab section number. You will be using the same rats and frogs for the next four lab periods. So, when you’re through with today’s lab, put the rats and frogs (along with any body parts you have removed) into the bag, and put it in the refrigerator. Next week you will retrieve the bag, and continue dissecting the same animals. Comments on Dissection: 1) The objective of a dissection is to produce a clear display of the system under investigation. For example, a good dissection exposes hidden body structures for observation so that another person could examine the specimen and see the essential relationships of the organs with the other organs without any difficulty. 2) At the onset, a scalpel or a pair of scissors is necessary to open the body cavity. Following this, most dissecting should be done with a pair of forceps, probe, or dissecting needle. It is necessary for you to carefully separate organs and pick away surrounding connective tissue. 3) Learn to use dissection guides, charts and other audiovisual materials to help you open up the body cavity and expose the various systems. These will be available in the lab. 4) Perform all dissections with a partner. 5) Once you have completed your dissection, ask the instructor to check it over with you. 6) Both the frog and the rat will be used to examine other systems. Attach an identifying label to your specimens and return them to the container. 49 1) The Earthworm A. Examine the worm model on display and identify each of the following parts in the model. I. buccal cavity II. pharynx III. esophagus IV. crop V. gizzard VI. intestine VII. calciferous glands B. Obtain a prepared slide showing a transverse section through a worm’s alimentary canal. Not the typhlosole. What is a typhlosole? What is the function of the typhlosole? C. Obtain a large worm from the plastic bucket where they are stored. Estimate how long the body is from mouth to anus. Estimate how wide the worm’s body is. Estimate how long the intestine of the worm is. Calculate a ratio for the length of the intestine divided by the length of the worm’s body. (Return the worm to the plastic bucket.) 2) The Frog A. Study the oral cavity of the frog. Open the mouth very wide. You may need to cut the muscles at the angle where the two jaws join together. On the upper jaw locate a row of small maxillary teeth. Notice the tongue in the lower jaw. Lift it up and find its point of attachment. At the posterior region of the oral cavity locate the opening to the esophagus. B. Open up the body cavity and locate the following parts of the digestive system: I. esophagus II. stomach III. duodenum IV. ileum (small intestine) V. large intestine VI. anus VII. cloaca VIII. liver IX. gall bladder X. bile duct XI. pancreas 50 C. Using your dissecting tools, remove the stomach and intestines from the frog and stretch them out. Again, estimate the length of the frog’s body and the frog’s intestine (when it is stretched out), and calculate a ratio for the length of the frog’s intestine divided by the length of the frog’s body. (Place the frog and any parts you have removed from it in the plastic bag you have prepared.) 3) The Rat A. Study the oral cavity of the rat. Examine the teeth and compare them to the teeth in the skulls of the other herbivores on display in the lab, as well as to the teeth of the cat, which is a carnivore. B. Open up the body cavity and locate the following parts of the digestive system: I. esophagus II. stomach III. duodenum (connected to stomach) IV. jejeunum (connected to the duodenum) V. ileum (connected to the jejeunum) VI. cecum VII. large intestine (colon) VIII. hepatic portal vein IX. liver X. gall bladder XI. pancreas XII. spleen 51 The following diagram may help: C. When you’re confident that you know where all these structures are, flag down the instructor and let the instructor quiz your group about which is which. D. Locate the rat’s liver, and determine how many lobes it has. E. As with the frog, use your dissecting tools to remove the stomach and intestines from the rat, and stretch them out. (Warning: Before removing the rat’s intestnes, make sure you find the hepatic portal system and hepatic portal vein. You will need to identify these in the lab on circulatory systems.) Estimate the body length of the rat, the length of the intestine when stretched out; and again calculate an intestine length to body length ratio for the rat. (When you are finished, place the rat and any parts you have removed from it in the bag.) 4) The Dogfish Shark, Rabbit and Cat A previously dissected dogfish and rabbit are on display, as well as the skeleton of a cat. Using the charts and dissection guides available, examine the components of the digestive systems of these organisms. Consider the type of food that each animal eats and look for specialized adaptations and differences between their digestive systems. 52 The dogfish is a close relative of the shark and the stingray, all of which are examples of what are commonly called cartilage fish. They are so named because they have a skeleton made of cartilage, rather than ossified bone. The cartilage fish often have an intestinal structure called a spiral valve. The spiral valve is shaped like a corkscrew. Locate the spiral valve on the dogfish. What do you think the purpose of the spiral valve is? 5) The Human On display is a human anatomy model. Examine the parts of the digestive system with the help of the diagram in your text. Observe the structure of the colon (large intestine) and it’s coiling nature. Compare the colon of the human to that found in the rat, rabbit and cat. Locate the following structures in the human anatomy model. (Make sure you can identify these structures in the model. You may have to do this in the lab exam.) I. Teeth II. Tongue III. Salivary glands IV. Esophagus V. Esophageal sphincter VI. Cardiac sphincter VII. Stomach VIII. Liver IX. Gall bladder X. Pyloric sphincter XI. Duodenum XII. Small intestine XIII. Large intestine 53 Part C: To Be Handed In Next Session. (5 points) Lab 6: Lab Report Name: CC Student Number: Course ID Number: Answer the following questions: 1. One of the recurring themes of biology is that several types of membranes, and several types of tissues are important for life. The larger the surface areas of these membranes are, the better the organism is at staying alive. However, organisms must often find a way to pack a large membrane surface into a small space. How do they usually accomplish this? 2. List at least three examples of tissues in the human body that are packed in this way. 3. What is the purpose of breaking food into smaller pieces before sending it through the alimentary canal? 4. What structure is used to break the food into smaller pieces in: A. The worm B. The Dogfish C. The Frog D. The Rat 5. Based on your answer to the above question, is the digestive system of a bird more like that of a rat or a worm? Explain your answer. 6. What is a spiral valve, and which sorts of organisms have one? 7. What was the intestinal length to body length ratio for: A. The worm B. The Frog C. The Rat 8. List the intestine to body length ratios for the three animals you measured. How are the rat and the frog able achieve such large intestinal length to body length ratios compared to the worm? (This isn’t a trick question. The answer is fairly simple.) 9. The spiral valve, the typhlosole (in the worm), and the coiled intestine (in the rat and frog) are adaptations made by three different types of organisms in order to achieve the same goal. What is this goal? 10. How many lobes did your rats liver have? 11. How many lobes does the human liver have? 12. ODONTOLOGY 1: Odontology is the study of teeth. List one basic way that the rats teeth are different from the dog fish and frog teeth? 13. ODONTOLOGY 2: Can you list the names of two different types of teeth that the rat has? 14. ODONTOLOGY 3: Can you list the names of two different types of teeth that the dogfish has? (Yes, this IS a trick question.) 54 15. ODONTOLOGY 4: List the main differences that you would expect to see in the teeth of a carnivorous mammal vs. an herbivorous mammal. 55 LABORATORY EXERCISE 7: Circulatory Systems Laboratory Exercise 7: Circulatory Systems In this lab you will examine the circulatory systems of the frog and rat, and also the hearts of cows, pigs and sheep. You will also be given the opportunity to check your blood pressure using both an old fashioned manual sphygmomanometer and a modern automatic one. You will also check your blood pressure using an automatic sphygmomanometer that is designed to fit around your wrist. You will also learn about several examples of congenital heart defects. Part A: Pre-Lab Quiz Questions. (5 points) Lab 7 Pre-Lab Quiz Questions 1. In your own words, explain the difference between an open and a closed circulatory system. Give an example of an animal with an open circulatory system, and an example of an animal with a closed circulatory system. 2. In your own words, explain how the material that carries oxygen to cells in the open circulatory system of an insect is different from the material that carries oxygen to the cells in a closed circulatory system of a mammal. (Hint: it has to do with how the material is packaged.) 3. In this lab you will be studying the circulatory system, and you will have to remove the rat’s thymus in order to see the heart’s aorta. However, the thymus is not part of the circulatory system. Which system is the thymus a part of, and what is its function? 4. What is a VSD? (Answer is in the lab protocols) 5. What is an ASD? (Answer is in the lab protocols) 6. What is ToF? (Answer is in the lab protocols) 7. What is PDA? (Answer is in the lab protocols) 8. What is the Foramen Ovale? (Answer in lab protocols) 9. What is the Ductus Arteriosus? (Answer in the lab protocols) 56 Part B: Lab Protocols. Circulatory systems pass, among other things, nutrients, gases, hormones, and blood cells to and from the cells of the body to help fight disease and help stabilize the body’s environment. In some organisms like the arthropods and mollusks, the blood bathes the tissues of the body (open circulatory system) while in others like vertebrates, the blood is strictly confined to a network of blood vessels (closed circulatory system). In this lab we will focus on the closed circulatory system of vertebrates. 1) The Frog A. Obtain your frog from last week’s digestive system dissection. B. Move the organs of digestion to one side and locate the dorsal aorta – the major artery lying on the dorsal wall. Trace the vessel towards the heart and determine from which chamber of the heart it originates. C. With a scalpel, carefully cut open the heart longitudinally, being careful not to damage any of the blood vessels connected to the heart. (How many chambers does the frog’s heart have? Is the septum complete, or incomplete?) D. With the help of diagrams and dissection guides available trace a blood vessel from the heart to one of the lungs; this vessel is a pulmonary artery. E. Be very careful separating the blood vessels in this area and you may be able to locate a blood vessel leaving one of the lungs; this is a pulmonary vein. F. Next, try to trace the complete circuit of the venous system. A large vein, the posterior vena cava, drains much of the body and transports this blood to the heart. G. Locate its origin between the kidneys from which it receives blood in a series of small renal veins. H. The hepatic vein enters the posterior vena cava in the region of the liver. I. Trace the posterior vena cava further into the heart. (In which chamber of the frog’s heard does the vena cava terminate?) J. Blood from the hind legs of the frog may return to the heart by either of two routes. Part of it passes forward to the kidneys in the renal portal vein while the remaining portion flows into the pelvic vein that passes to the midline in the ventral wall of the abdomen and unites with the pelvic vein from the other leg to form the ventral abdominal vein. K. Trace this vessel to the liver. L. In the liver locate the hepatic portal vein that collects blood from the intestines. 2) The Rat A. Obtain your rat from last week’s digestive system dissection. B. Carefully cut the sternum (breast bone) and peel the ribs back to expose the thoracic cavity, containing the heart and lungs. You may find the following diagram helpful. 57 C. Locate the heart and remove the thymus, the large lymphoid organ that covers most of the blood vessels that enter and leave the heart. D. Trace the complete circuit of the arterial system as far as you can with the help of the dissection guides. E. You should be able to locate the following structures: dorsal aorta, right and left pulmonary arteries, celiac artery, mesenteric artery, renal artery, and right and left iliac arteries. F. Similarly, trace the complete circuit of the venous system by locating the following vessels: anterior and posterior vena cava, right and left pulmonary veins, hepatic portal vein, renal veins and iliac veins. You may find the following diagram helpful: G. When you feel you’re ready, call your instructor over, and identify the following structures to him/her: A. Aorta B. Left and right vena cavae C. Brachicephalic artery 58 D. E. F. G. H. I. J. K. L. M. N. O. Left and right atria Coronary veins and arteries (on the heart) Common carotid artery (may not be visible if you didn’t dissect the neck area) Subclavian artery Axillary artery Brachial artery (may not be visible if you didn’t dissect the arm) Thoracic aorta Abdominal aorta Anterior mesenteric artery (supplies blood TO the intestines) Hepatic portal vein (returns blood from the intestines to the liver) Renal arteries (send blood to the kidneys) Renal veins (return blood from the kidneys) 3) The Anatomy of the Mammalian Heart In this exercise we will be using preserved hearts of cows, pigs and sheep. The human heart is very similar in construction. External Anatomy of the Mammalian Heart A. Examine the heart and using your fingers, apply pressure to both sides. Determine which side is more muscular and considering this difference, determine which is the right side and which is the left side of the heart. B. From this, determine the dorsal and ventral surfaces of the heart. C. Locate the coronary vessels and trace them to their source of origin as far as you can. D. Locate the four chambers of the heart: left atrium, right atrium, left ventricle and left atrium. Notice the dramatic size difference between an atrium and a ventricle. E. Look at the ventral side of the heart and locate the two types of major veins connected to the heart - the anterior and posterior vena cavae and the pulmonary veins. F. Insert your fingers into these veins to trace their connections. G. Insert your fingers into the veins and feel their thickness. Which do you expect to be thicker, veins or arteries? Can you see any of the valves? (You should be able to at least locate the pulmonary semilunar and aortic semilunar valves.) H. Look at the dorsal side of the heart and locate the two types of major arteries connected to the heart – the aorta and the pulmonary arteries. Use your fingers to feel the thickness of the arterial walls. Internal Anatomy of the Mammalian Heart I. Examine one of the hearts that are cut into two longitudinal halves. J. Locate the four chambers, noting again the reasons for distinguishing between the left ventricle and right ventricle. 59 K. Note the muscles that completely separate the four chambers from one another. L. Locate the four valves: tricuspid valve, bicuspid valve, pulmonary semilunar valve and aortic semilunar valve. Note the position of each valve as this will help you in determining their function. M. Locate the chordae tendinae attached to the tricuspid and bicuspid valves. N. Trace the pathway of the blood through the heart as part of a complete cardiac cycle. O. Make sure you can identify (with reasons) and give functions for the chambers, veins, arteries and valves of the heart. 4) Anatomy of The Human Heart Examine the human anatomy models on display (heart and torso models), and make sure you can identify the following structures: A. Left and right lungs (which has 3 lobes?) B. Intercostal muscles C. Heart D. Aorta E. Left and right atria F. Left and right ventricles G. Superior and inferior vena cava 5) Congenital Heart Defects in Humans: Observe the model of the normal human heart, as well as the five models showing various types of congenital heart defects labelled A through E. Study these models, because you might be asked about them on the lab exam. You have learned in lecture that the human heart has four chambers (two atria and two ventricles) separated by walls called septa (singular: septum). A “septal defect” is a hole in one of the septa which allows oxygen poor blood from the left side of the heart to mix with oxygen rich blood in the right side of the heart. There are two general types of septal defects. An Atrial Septal Defect (ASD) is a hole in the septum that separates the left and right atria. A Ventricular Septal Defect (VSD) is a hole in the septum that separates the left and right ventricles. ASDs are more common than VSDs, and are often caused by a failure of the fetal heart to develop properly. Inside the womb, a developing fetus does not have access to air to oxygenate its own blood. Instead, it gets oxygen from its mother via the placenta and umbilical cord, and the developing fetal lungs and heart are bypassed. Oxygenated blood from the umbilical cord flows into the right atrium from a spot where the umbilical cord is attached to the inferior vena cava. A hole in the fetal atrial septum, called the foramen ovale, allows the blood to flow 60 directly from the right atrium to the left atrium, bypassing the fetal lungs. The foramen ovale is supposed to close when the baby is born, but sometimes it remains partly or completely open. When the foramen ovale fails to close it creates a condition called Patent Foramen Ovale (PFO). (The word ‘patent’ means open, or unclosed.) Types of ASDs: If the atrial defect is located up near the area where the superior vena cava empties into the right atrium it is called a “Sinus Venous ASD.” If it is located lower down, but still closer to the back of the right atrium than middle, it is called an “Ostium Secundum ASD,” and if it is located directly between the two atria it is called an “Ostium Primum ASD.” (See Figure 1.) FIGURE 1: Types of Atrial Septal Defects (ASDs). 1. Sinus Venous ASD 2. Ostium Secundum ASD 3. Ostium Primum ASD 61 Types of VSDs: VSDs are less common than ASDs, but like the ASDs they are given different names depending on where the defect is located. Refer to Figure 2 for how the VSDs are named. FIGURE 2: Types of Ventricular Septal Defects (VSDs) 1. Perimembranous VSD 2. Muscular VSD 3. Canal Type VSD 4. Subpulmonary VSD Other Types of Congenital Defects: In addition to the foramen ovale, there is another hole that is normally present in the developing fetal heart that connects the pulmonary artery directly to the aorta. This connection between the pulmonary artery and the aorta is called the ductus arteriosus, and it is supposed to close before the baby is born. If it does not, it leads to a condition called patent ductus arteriosus (PDA). Finally, when VSD is combined with a narrowing of the pulmonary artery, it leads to a condition called tetralogy of Fallot (ToF). ToF is a very serious condition, and is sometimes called “blue baby syndrome” because babies that are born with it appear blue, due to lack of oxygen in the blood (blue colouring due to lack of oxygen in the blood is called cyanosis.) The Assignment: The models are labelled A through E. One of the models shows ToF, one shows PDA, one shows a VSD, and two show ASDs. Correctly identify which is which, and give the correct names to the two different types of ASDs and the single VSD. (Submit your answers in your lab report.) 62 6) Measuring Blood Pressure Measuring blood pressure with the manual sphygmomanometer: It is possible to do this by yourself, but easier if you allow your friend to do it for you. You can then switch roles, and measure your friend’s blood pressure. (You’ll have to roll up your sleeve. The pressure cuff can’t effectively cut off the blood flow if you’re wearing a shirt.) A. Wrap the blood pressure cuff around your upper arm, above the elbow. B. Place the stethoscope on the brachial artery, underneath the pressure cuff. C. Screw the valve on top of the squeeze bulb shut, and inflate the cuff until you can no longer hear the thumping sounds (the Korotkoff sounds). (Note: you won’t hear anything as you begin to inflate the cuff. Then you’ll hear the Korotkoff sounds, and then you’ll stop hearing them again. Stop inflating the cuff as soon as you hear the sounds stop, otherwise you’ll cause pain in the arm. Generally, you shouldn’t have to inflate the cuff beyond about 190. If you do, it probably means that you’ve placed the stethoscope in the wrong place.) D. Once you’ve stopped hearing the Korotkoff sounds, unscrew the valve at the top of the squeeze bulb and SLOWLY let the air out of the cuff. E. When you START hearing the Korotkoff sounds again, record the pressure. This is the systolic pressure. Continue letting the pressure drop. F. When you STOP hearing the Korotkoff sounds again, record the number. This is the diastolic pressure. G. The normal systole/diastole ratio for a young person is approximately 120/80. Do not be alarmed if your pressure deviates somewhat from this. Measuring blood pressure with the automatic sphygmomanometer: Simply wrap the cuff around your brachial artery, just above the elbow, and push the “Start” button. Record the value you got, and compare it to the value you got from the manual sphygmomanometer. Measuring blood pressure with a WRIST sphygmomanometer: This sphygmomanometer is meant to be wrapped around your wrist. You simply wrap it around your wrist with the control panel on the palm side of your hand, and press the “Start” button. 1. Measure your blood pressure with your hand held ABOVE YOUR HEAD. Record the value. 2. Measure it again, with your hand held at the same level as your heart. Record the value. 3. Measure it again, with your hand held down by your hips. Record the value. 63 Part C: To Be Handed In Next Session. (5 points) Lab 7: Lab Report Name: CC Student Number: Course ID Number: A. Draw a ‘circuit diagram’ (a schematic diagram) of the rats circulatory system including the following structures: 1. Heart 2. Left and right lungs (pulmonary circuit) 3. Head (cephalic circuit) 4. Abdomen (systemic circuit) B. Congenital Heart Defects: Identify which defect each of the heart models had. A. B. C. D. E. C. Answer the following questions: 1. How many lobes did the rat’s right lung have? 2. How many lobes did the rat’s left lung have? 3. How many lobes did the frog’s right lung have? 4. How many lobes did the frog’s left lung have? 5. How many chambers did the frog’s heart have? 6. Did the frog have a complete septum? 7. How many chambers did the rat’s heart have? 8. Did the rat have a complete septum? 9. Generally speaking, the frog’s lungs are smaller than the rats (relative to body size). How is a frog able to survive with smaller and less efficient lungs, as well as a less efficient circulatory system than a rat? 10. What are the functions of the following blood vessels in the rat (as well as other mammals)? D.Blood Pressure: 1. What was your blood pressure as measured by the automatic sphygmomanometer? 2. What was your blood pressure when you measured it with the wrist sphygmomanometer when you held your hand: A. Above your head: B. On the same level as your heart: 64 C. Down by your hip: 3. When you measured your blood pressure with the wrist cuff, you probably got three different blood pressures. Where was the blood pressure GREATEST? Can you suggest a reason why it would be greatest at that spot? 65 LABORATORY EXERCISE 8: The Respiratory System Laboratory Exercise 8: The Respiratory System In this lab you will examine rat and frog lungs under the dissecting microscope, and also the gills used by fish and squid. Rat and frog lungs are adapted for extracting oxygen from air, while gills are adapted for extracting oxygen from water. You will also measure your lung capacity using a spirometer, and calculate the average male and female tidal lung volumes for your class. Part A: Pre-Lab Quiz Questions. (5 points) Lab 8 Pre-Lab Quiz Questions 1. 2. 3. 4. 5. 6. 7. 8. What is a spirometer, and what does it measure? What is tidal breathing? What is the tidal volume of lungs? What is the residual volume of lungs? What is the average tidal volume for males and females? What is the average respiration rate? What is the average concentration of oxygen in air vs. water? Based on your answer to the above question, which do you think is more efficient at extracting oxygen from its environment, lungs or gills? 9. What is a countercurrent gas exchange system? 10. Which of the following organisms uses a countercurrent gas exchange system, fish or mammals (ie-rats)? 11. Do frogs use a countercurrent gas exchange system? 12. Why do most frogs have to live in or very near the water? (Hint: it has to do with gas exchange.) 66 Part B: Lab Protocols. All animals need to take in oxygen and eliminate carbon dioxide. Lungs are membranous structures adapted for gas exchange in a terrestrial environment, and gills are membranous structures adapted for gas exchange in an aquatic environment. Oxygen must be dissolved in water before animals can take it up and therefore the respiratory surface of animals must always be moist. Very small organisms do not need respiratory surfaces because they have a high surface area to volume ratio. Animals use a variety of structures to enhance the exchange of respiratory gases. These include skin, gills, tracheal systems, and vertebrate lungs. In this lab we will examine the characteristics of these respiratory organs. 1) Gills Gills provide a large surface area for gas exchange in aquatic organisms. It is difficult to circulate water past gills because water is dense and the oxygen concentration in water is low. To circulate the water past the gills, amphibian larvae physically move their gills, mollusks pump water into the mantle cavity which contains the gills, and some crustaceans gills are attached to branches of the walking legs. The flow of blood in fish gills is opposite the direction that the water passes over the gills. This arrangement, called countercurrent flow, enables fish to extract more oxygen from the water than if blood moved in the same direction as the passing water. Squid: The squid is a marine cephalopod of the Phylum Mollusca (along with snails, slugs, clams etc.) with a pronounced head, bilateral symmetry, eight arms arranged in pairs, and two longer tentacles. A mantle surrounds the viscera of the organism, located above the head. A. Examine the dissected squid. B. The mantle has been cut open to display the gills lying in the mantle cavity. C. Answer the following question: how does the squid facilitate passing water over its gills? Compare this method to how a fish facilitates passing water over its gills. Fish: The term fish includes a number of different aquatic animals in the Phylum Chordata, Subphylum Craniata, that possess bilateral symmetry, a streamlined body, and scales. Organisms such as hagfishes and lampreys (jawless fish) are included with the more familiar cartilaginous fish, the ray-finned bony fish, and the lobe-finned bony fish. Modern representatives of fish display greater diversity that any other group of vertebrates. A. Examine the head of a fish on demonstration. 67 B. With the operculum removed, count the number of gills present on each side of the mouth. C. Carefully remove one gill and locate the gill arch, gill rakers and branchial filaments. See the diagram below for assistance in locating these structures. 2)Lungs Simple lungs evolved some 450 million years ago in fish. Some evolved into swim bladders and other evolved into more complex structures we call lungs. Paired lungs are the respiratory surfaces in all reptiles (including birds) and mammals. The amphibian lung is a simple, convoluted sac. The lungs are small and do not contribute to the majority of gas exchange. Instead, the skin performs most of the respiration in these animals. Reptiles and mammals do not use the skin as a primary respiratory surface and instead possess lungs with numerous alveoli. Diffusion of gases occurs across the alveolar surface. RAT A. Obtain your specimen of the rate used in the digestion and circulatory system labs. B. Open the mouth of the rat wide enough to expose the tubes leaving the pharynx by cutting through the skin, muscle and bone on both sides of the jaw. C. Locate the external nostrils and trace the pathway of air from here into the pharynx. 68 D. Along the neck region, locate the trachea. Note the position of the trachea relative to the esophagus and note the differences in their structures and how these differences correlate to their functions. E. Follow the trachea in a posterior direction until it branches into two bronchi, each of which enters one of the two lungs. F. Make a longitudinal cut through one lung to see the branches of a bronchus. G. Examine the dissected rat lung under the dissecting microscope. Can you see the alveolae? H. SKETCH what you see, and include it with your lab report. FROG: A. B. C. D. E. Obtain your frog. Remove one lung, and make a lateral incision, cutting it in half. Examine it under the dissecting microscope. Can you see the alveolae? Which type of lung, rat or frog, has the greater amount of alveolae per unit area? SKETCH what you see, and include it with your lab report. PIG: A. Observe the fresh lung of a pig and observe its texture. B. Locate a bronchus and trace it as far as you can by carefully cutting along its path. C. Appreciate the fine structure of the lung; a series of tubes terminating in microscopic sacs called alveoli. D. Locate the various divisions of the bronchi: primary, secondary and tertiary. HUMAN: Observe the human torso model and trace the pathway of oxygen from the air through the notrils and to the alveoli. 3) The Lung Capacity of Humans The amount of air in the lungs at any given time can be measured in several ways. Total Lung Capacity is the amount of air in the lungs after a deep inhalation; it is equal to the Vital Capacity plus the Residual Volume. Vital Capacity is the amount of air exhaled in one breath – the maximum amount of air that can be forcibly exhaled after breathing in as much as possible. Residual Volume is the amount of air left in the lungs after a 69 deep exhalation. Tidal Lung Capacity is the amount of air your lungs hold during normal breathing – the amount of air moved in and out of the body in one breath. Lung volumes differ with age, sex, body frame, and aerobic fitness. Measuring your lung capacity can help you determine how much stamina you have available to go about your daily routine, including sports and other activities. Usually you need about one-third of your lung capacity to carry out routine tasks that do not require exertion. It is also possible for you to increase your lung capacity through regular exercise. Volume (capacity) is measured in liters (l), milliliters (mL), and cubic centimeters (cm3). One milliliter is equal to one cubic centimeter and 1,000 milliliters is equal to one liter. Measuring Vital Capacity: A. Obtain an unused paper tube to blow into the spirometer. (Do not put your lips directly onto the plastic.) Once you have finished measuring your lung capacity you can dispose of the cardboard tube in the regular garbage. B. Make sure that the spirometer is ‘zeroed.’ The person who used it before you might have forgotten to reset it. C. Using the spirometer, take as deep a breath as possible and exhale as deeply as possible into the spirometer. D. The expiration should not occur in a series of sudden spurts, but as one long, continuous breath. E. Do this twice and use the higher figure. RECORD THE VITAL CAPACITY OF EVERYBODY IN THE LAB. CALCULATE THE AVERAGE VITAL CAPACITY FOR MALES AND FEMALES 70 Part C: To Be Handed In Next Session. (5 points) Lab 8: Lab Report Name: CC Student Number: Course ID Number: Include the sketches you made of the frog and rat lungs when viewed under the dissecting microscope. Indicate which you thought had the greater degree of infolding and surface area. Answer the following questions: 1. If the fish gill system is more efficient than the rat (or human) lung system, due to countercurrent exchange, why can’t a fish breath air? 2. Which organism relies on elastic recoil to breath, mammals or fish? 3. How does the fish facilitate water passage over its gills? 4. How does the squid facilitate water passage over its gills? Include all of your lung capacity data, and calculate the average lung capacity for males and females in your lab. 71 LABORATORY EXERCISE 9: The Renal and Reproductive Systems Laboratory Exercise 9: The Renal and Reproductive Systems Part A: Pre-Lab Quiz Questions. (5 points) Lab 9 Pre-Lab Quiz Questions 1. What is the basic functional unit of a kidney? (Hint: it’s microscopic.) 2. Which part of the human kidney has a higher concentration of urea, the renal cortex or the renal medulla? 3. Where is urine collected in the human kidney before it enters the ureter? 4. What is the name of the microscopic tubes in which sperm cells are formed in a teste? (Answer in the lab protocol.) 5. When you are dissecting a female rat, or any other female mammal, what is one easy way to distinguish the uterus from the urinary bladder? (Answer in the lab protocol.) 72 Part B: Lab Protocols. I. The Renal (Kidney) System: Cells produce water and carbon dioxide as by-products of metabolic breakdown of sugars, fats, and proteins. Chemical groups such as nitrogen, sulfur and phosphorus must be stripped from the large molecules to which they are attached as part of preparing them for energy conversion. The continuous production of metabolic wastes establishes a steep concentration gradient across the plasma membrane, causing wastes to diffuse out of cells and into the extracellular fluid. Single-celled organisms have most of their wastes diffuse out into the outside environment. Multicellular organisms, however, and animals in particular, must have a specialized organ system to concentrate and remove the wastes from the interstitial fluid. These wastes are moved into the blood capillaries and eventually deposited at a collection point for removal entirely from the body. Excretory systems serve this purpose. In animals, this system regulates the chemical composition of body fluids by removing metabolic wastes and retaining the proper amounts of water, salts, and nutrients. MAMMALIAN KIDNEYS The Rat: A. Obtain your rat from last time. B. With the help of the following diagrams, identify the following structures. (You may have to cut further down into the abdomen than you already have to expose these structures.) I. II. III. IV. V. VI. VII. VIII. a. Abdominal aorta Renal arteries Renal veins Kidneys Ureters Urinary bladder Ovaries (female) Uterus (female) IX. X. XI. Seminal vessicles (male) Prostate gland (male) Vas deferens (male) 73 C. Determine whether your rat is a male or a female. Make sure you go to one of the other tables where they have a rat of the opposite sex, and examine their rat as well. D. When you are confident that you can identify all of these structures, call your instructor over to your bench, and get him/her to quiz you on them. E. Remove one of the rat’s kidneys an slice it in half, longditudinally. F. Remove the other kidney and slice it transversely. G. Look at both under the dissecting microscope, and see if you can distinguish the renal cortex from the renal medulla. H. Look at the longditudinally sliced kidney under the dissecting microscope, and see if you can distinguish any renal pyramids. If you can, show them to your instructor for verification. Kidneys of Larger Animals: Observe the dissected pig kidneys, and the model of a human kidney (actual size). Be sure you can distinguish the renal cortex, the renal medulla, the renal pelvis, and the ureter. You may be asked to do this on the lab exam. URINALYSIS Urinalysis: Many human diseases can be diagnosed via abnormalities in urine. In this week’s lab you will be analyzing four samples of artificial urine to look for indications of disease. You will begin by analyzing the specific gravity of the urine samples, and then move on to test a number of other factors using something called a urine test strip. The urine test strip analyzes several things simultaneously, including the pH of the urine, as well as the relative concentrations of carbohydrates, ketones and proteins in the urine. Before using one of these (very expensive) test strips to analyze urine, you should understand what the tests mean. Using the information contained in the Wikipedia pages on urinalysis, the urinometer, and test strips, answer the following questions in your own words. Put the answers in your lab report. 1. What is the ‘specific gravity’ of a solution, and how is it related to the concentration of solute dissolved in a solution? 2. What is the normal range for the specific gravity of urine? 3. What is the normal range for pH of urine? 4. What is glycosuria? 5. What is the normal range of glucose concentration found in urine? 6. Would you expect a person suffering from diabetes mellitus to have a higher or a lower than normal concentration of glucose in their urine? 7. What is ketonuria (also known as ketosis)? 8. What are ketone bodies, and how are they related to diabetes mellitus? 9. What is the normal range of protein found in urine? 10. The nephron is not supposed to let proteins be filtered out of the blood and into urine. If excessive protein is found in urine it could be an indication that the kidneys are under stress from illness. What is the normal concentration range of protein found in urine? 11. Based on your knowledge of the most common proteins found in blood, which protein would most likely be found in urine? WARNING: the questions about the normal specific gravity, pH, glucose and ketone content of human urine might be on the lab exam. So, make sure you know what they are. The Wikipedia pages can be found here: http://en.wikipedia.org/wiki/Urinalysis http://en.wikipedia.org/wiki/Urinometer http://en.wikipedia.org/wiki/Urine_test_strip PROTOCOL: Using a Urine Test Strip. A. Your group should measure only one of the four artificial urine samples, and then write your results on the board. Then copy the data from all four samples and use it to fill out the lab report. B. Each group should use ONLY ONE test strip. (They are expensive.) C. When you are measuring the specific gravity of your sample, be very careful when handling the urinometer! It is fragile, and contains mercury, which is very toxic! Do not break the urinometer! In order to measure the specific gravity, fill a graduated cylinder about 2/3 of the way to the top. Then, carefully lower the urinometer into the cylinder, and slowly let go of it. Do not drop the uninometer into the cylinder from a great height, or it will bounce off the bottom of the cylinder and break. Measure the specific gravity from the bottom of the meniscus. D. Please pour the urine sample back into the bottle for the next lab group to use. E. Wash the urinometer very carefully with warm water, and put it back into the box. II. The Reproductive System. 1. Obtain one of the testes from a male rat and make cut it in half, longitudinally. Examine it under the dissecting microscope. Using a probe (needle) pull out some of the seminiferous tubules, where the sperm cells are formed, and observe them under the microscope. 2. Obtain one of the ovaries from a female rat and cut it in half. (When dissecting out an ovary, you may have some trouble distinguishing the urinary bladder from the uterus. The easiest way to tell them apart is to follow the ureters down from the kidneys, noting that the ureters lead to the bladder and not the uterus. Therefore, the uterus will be the OTHER thing.) Observe the ovary under the dissecting microscope. Can you see a corpus luteum? 75 3. Remove the uterus from a female rat, cut it in half, and observe it under the microscope. Can you distinguish the endometrium from the muscular tissue? 76 Part C: To Be Handed In Next Session. (5 points) Lab 9: Lab Report Name: CC Student Number: Course ID Number: Print out the lab data for the artificial urine samples, and conclude which samples might be from a patient with diabetes mellitus. Explain your reasoning by comparing the numbers obtained to the numbers you would expect from a normal urine sample. 77