Lab - The Gold Rush
advertisement

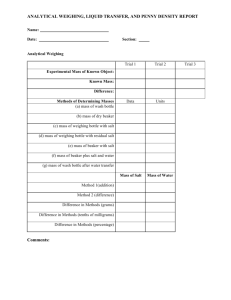
DO NOT WRITE ON LAB Lab – Alchemy (aka The Magic Penny) Goggles must be worn at all times! Introduction: Long ago, early scientists, tried to turn ordinary things into gold. This pursuit was called alchemy and the people who engaged in alchemy were called alchemists. This laboratory experiment is similar to the experiments done by alchemists hundreds of years ago in their search for the key to making gold. Purpose: In this activity you will determine whether it is possible to turn copper into gold. Materials: pennies 3 Beakers, 150-mL 2 Sodium chloride, 2.5–3 g Graduated cylinder, 50-mL Ring Stand Vinegar, 15 mL Bunsen Burner Zinc chloride solution, 1 M, 25 mL Tongs Zinc, granular, 1.0 g Towel or paper towel Balance Water, distilled Procedure: 1. Weigh out and place 2.5–3 g of sodium chloride and 15 mL of vinegar in a clean, 150-mL beaker. 2. Clean two pennies by placing them in the sodium chloride/vinegar solution until they are shiny. 3. Remove the pennies using tongs and rinse thoroughly with water. Dry thoroughly with a towel. Note: Do not handle the clean pennies with your hands. The oils from your skin may interfere with the reaction. 4. In a clean 150-mL beaker, mix together 1.0 g of granular zinc and 25 mL of 1 M zinc chloride solution. (Skip step if you are re-using a previous group’s solution). 5. Place the beaker with the ZnCl2 and zinc on a ring stand over a Bunsen burner. 6. Carefully and gently heat the mixture until the solution boils. 7. Using tongs, immerse two pennies side by side in the boiling mixture until their appearance changes. 8. Use tongs to remove the pennies. Caution: The pennies will be very hot. 9. Carefully dip the pennies into a beaker of distilled water. Shine the pennies with a towel. Set one treated penny aside to be used for later comparisons. 10. Using tongs, place the other treated penny over the Bunsen burner flame until the penny changes color again. Using a heat-resistant glove or tongs, flip the penny every 5 seconds to avoid burning. 11. Use tongs to remove the penny from the heat and immediately dip the penny into a fresh beaker of distilled water. The penny will be extremely hot and should be handled with tongs until it has cooled for several minutes. 12. SAVE the ZnCl2 solution for future classes. DO NOT WRITE ON LAB Data/Observations: Penny 1 Penny 2 Year of the penny What color did the penny turn when boiled in the zinc chloride solution? What color was the 2nd penny upon heating? N/A Calculations/Analysis: 1. Using your periodic table fill in the following information on your own paper: Element # protons # electrons # neutrons Copper Silver Gold 2. What atomic characteristic makes: a) Gold unique from all other elements? b) Silver unique from all other elements? 3. a) Did you actually turn copper into silver & or gold? Explain your reasoning. b) If you really change copper into gold, what would you have to do to the atomic structure of the copper? Discussion Questions: 1. If you were a merchant a hundred years ago, would you accept “gold” as payment from an alchemist? Explain 2. How could you test the “gold” to see if it was real? (you will need to use outside resources for this question) Conclusion: Write a minimum of 3-4 quality sentences restating the purpose of the lab. In addition you can discuss your results, what you learned from the lab, and any errors you made during the lab that you’d correct in the future.