water analysis
advertisement

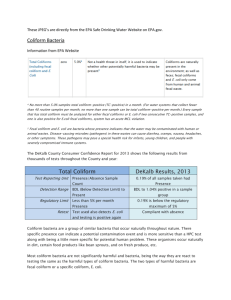
WATER ANALYSIS THIS LAB REQUIRES YOUR TABLE TO BRING A WATER SAMPLE TO CLASS— aquarium water, toilet water, pond water, sewage water, etc., but not tap water. Water supplies have to be constantly monitored for a variety of materials----bacteria, nitrates, pesticides, metals, etc. In this lab, you will analyze water for bacteria, and, in particular, an indicator group of bacteria called the coliforms. Although you will be looking at the total counts of aerobic bacteria in the water sample that YOU BRING TO LAB, the coliform bacteria are the critical test organisms which are found in improperly treated water or water that has fecal contamination. They have been used as such for most of the 20th century and still today. These indicator bacteria, such as E. coli, are easier to look for than the professional pathogens such as Listeria, Salmonella, or Vibrio. E. coli is easy to grow and is typically associated with feces. Another group of indicator bacteria, the fecal streptococci (enterococci), are also identified in water. The indicator bacteria are generally not associated with pathogenicity. Coliforms are bacteria that are naturally occurring in animals and in the environment: they are indicators of other potentially harmful microorganisms in drinking water. They are all gram negative bacteria that ferment lactose, and are non-sporeforming. FECAL coliforms, exemplified by E. coli, indicate water contaminated with animal or human waste, i.e. feces. Microbes in the fecal-contaminated water may cause food-borne illness that has short-term symptoms—nausea, diarrhea, vomiting—or if severe enough may cause death. This is a real problem in the immunocompromised, immunodepressed, and babies and children. METHODS OF EVALUATING FOR BACTERIA Membrane filter technique: Filtering 100 ml of water through a millipore filter with holes smaller than the bacteria causes the bacteria to be trapped on top of the filter. The filter pad is then placed on special coliform media which allows a coliform count to be done. Most probable number: The water sample is diluted and inoculated into a variety of specialized fermentation media tubes having a lactose broth. The MPN is determined with the help of a standard chart, based on the number of broths that have turned positive (fermented by bacteria in sample). The results are given as number of coliforms per 100 ml of water. If coliforms are present, the lab will generally recommend that a second sample be analyzed. If the number of coliforms was over 30, a second sample is essentially useless. One or more coliform bacteria/100 ml = "does not meet the bacteriological standard purity" This lab incorporates a newer method of performing counts on bacteria, called Petrifilms from 3M corporation. There is more information—-interpretation and photos—-on these materials at the 3M website. Since the aerobic counts include all coliforms, the water sample will be diluted out. When the water sample is added to the dehydrated media, the water-soluble gel will rehydrate, forming a thin media plate of sorts. OBJECTIVES: Identify coliform bacteria using 3M Petrifilms. Differentiate between coliforms and fecal coliforms. Analyze water samples for bacterial counts. MATERIALS NEEDED: per table water sample 1 coliform/E. coli count petrifilm 2 aerobic count petrifilms 1 ml pipettes scissors 2-99 ml phosphate dilution containers humidified container to incubate petrifilm plates THE PROCEDURE: 1. Bring water sample to class, preferably a rather dirty sample—not tap water. 2. Prepare a dilution of the water sample by taking a 1ml aliquot and placing it into a 99ml phosphate buffer solution. This is the 1/100 dilution. 3. Using a fresh pipette, prepare a 1/10,000 dilution by taking a 1ml aliquot and placing it into another 99ml phosphate buffer solution. 4. Be sure the containers are shaken well. 5. Place the Petrifilm flat on the table. Lift top film. THE AEROBIC COUNT PETRIFILM: Place 1 ml of each dilution in the center of 2 petrifilm plates (already labeled with 1/100 and 1/10,000). THE COLIFORM COUNT PETRIFILM: Place 1 ml of the original water sample in the center of the petrifilm plate. 8. Release the top film and allow it to drop. 9. Using the small plastic spreader, place it over the inoculum. Gently apply pressure on spreader to distribute the sample over the circular area. Do not rotate or twist the spreader. For aerobic count petrifilm-- RIDGE side down, place spreader on top film over inoculum For coliform petrifilm--With FLAT side down, place spreader on top film over inoculum 10. Remove the spreader and wait at least 1 minute for the gel to form. 11. Incubate the plates with clear film side up in a humidifed container (1 for the entire class). You may stack the petrifilms. 12. Incubate the petrifilms at 30 degrees C for 48 hours. INTERPRETATION: 1. Count the colonies on a Quebec colony counter or other magnified light source (with clear films down). 2. Refer to the INTERPRETATION GUIDE in print form or at the 3M website given above. 3. After counting the clear film covers can be lifted, and the colonies can be used for testing or staining. AEROBIC COUNT PLATE: There is a red indicator dye in the media gel that colors the colonies. Count all red colonies of any size or intensity of red. COLIFORM COUNT PLATE: The pH indicator violet red is incorporated into the gel, along with bile which inhibits gram positive bacteria. In addition, there is an indicator of the enzyme glucuronidase which will turn blue if the bacterium makes the enzyme. You may also see carbon dioxide gas bubbles between the film and the bottom of the petrifilm. Most E. coli make both the glucuronidase and CO2, and those colonies will be blue or blue-red. Non-fecal coliforms will be red colonies. 3M Indicator Testing (click here to go to the information website) QUESTIONS: 1. What criteria are used to define the coliform group? 2. Why is there a dye added to the coliform petrifilms? 3. Why are coliforms used as indicator organisms for water impurity? Fall 2011 - Jackie Reynolds, Richland College, BIOL 2421