IUPAC RULES
advertisement

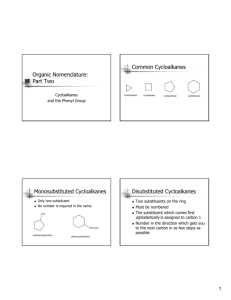
IUPAC RULES (Alkanes) Small Alkyl Chains (1C) methane; (2C) ethane; (3C) propane; (4C) butane; (5C) pentane; (6C) hexane; (7C) heptane; (8C) octane; (9C) nonane; (10C) decane; (11C) undecane; (12) dodecane, (13) tridecane; (14C) tetra- ; penta- ; hexa- ; hepta- ; octa- ; nona- ; (20C) eicosane Common Alkyl Fragments Structure of fragment Common name methyl IUPAC name methyl ethyl ethyl n-propyl propyl i-propyl (methylethyl) n-butyl butyl CH sec-butyl (1-methylpropyl) CH3 CH2 i-butyl (2-methylpropyl) t-butyl (dimethylethyl) isopentyl (3-methylbutyl) neopentyl (2,2-dimethylpropyl) H3C H3C CH2 H3C CH2 CH2 CH3 CH3 H3C CH CH2 H3C CH2 CH2 CH3 CH CH2 CH3 CH3 CH3 C CH3 CH3 CH CH2 CH2 CH3 CH3 CH3 C CH2 CH3 Abbreviations used are n- for normal, i- for iso; s- or sec- for secondary; t- or tert- for tertiary. Straight Chain Alkanes (1) Identify the longest continuous “parent chain” and assign it a base name • if 2 or more chains have the same longest length, the chain with the most substituents is the parent (substituent = group attached to but not counted as part of the longest chain) (2) Name all substituents (3) Number the parent chain (1,2,3…) beginning with the end nearest a substituent • • (4) List substituents (with number locations) in alphabetical order • (5) if the same set of numbers can be obtained in both directions, start numbering on the end with the substituent that comes first alphabetically if the same alphabetically with other substituents, choose the end with the lowest # for the 2nd substituent if the same group appears more than 1X, use prefixes di-, tri-, tetra- etc. instead of listing the same substituent multiple times. Prefixes are ignored in alphabetizing. (Note: The prefix iso- is used in alphabetizing because it is considered part of the alkyl group name, however, n-, t- and sec- are not used in alphabetizing.) With complex substituents, use () to set off the substituent name. The point of attachment is C’1 of the substituent Cyclic Alkanes (1) Identify the largest ring as the “cyclo parent” (2) If one substituent, name = substituent cyclo parent (no need to add a number) (3) If 2 substituents on different atoms, the #1 position is given to the substituent that is first alphabetically (4) If >2 substituents, cite in alphabetical order; substituent assigned #1 is the one that gives the smallest # for the next substituent (clockwise or counterclockwise does not matter) (5) If 2 substituents on the same carbon, and another substituent is on a different carbon, the carbon with two substituents is designated C1 (6) If the substituent consists of a longer chain or if more than one ring is present, a ring can be considered a substituent Bicyclic Alkanes (1) Count the total number of ring carbons and name the parent alkanes. Add the prefix “bicyclo” if it is a bridged or fused bicyclic alkane. (2) Consider all bicycloalkanes to have up to three “bridges” linking up to two “bridgehead” carbons. Indicate in [ ] the number of carbons in each bridge, starting with the largest one. (3) Insert the numbers in brackets into the name, after “bicyclo” or “spiro”. (4) Number the bicycloalkane starting at one bridgehead position. Count along the longest bridge to the other bridgehead, then count along the next longest bridge back to the first bridgehead, then finally count along the shortest bridge (if there is a third bridge). Place substituent names and position numbers as prefixes, as usual. CH3 CH2CH3