SPM form 4 chemistry chap 9 exercises - E
advertisement

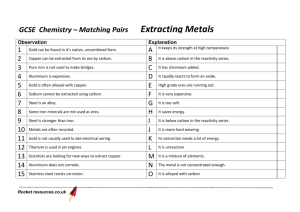
Akhmalazmi86@blogspot.com Form 4 Chapter 9 FORM 4 CHAPTER 9 MANUFACTURED SUBSTANCES IN INDUSTRY ANALYSIS OF PAST YEAR QUESTIONS FROM 2003 – 2008 Year Paper No. Type of question Question No 2003 P2 S 3 2004 P3 E S P2 E 3 S 2005 P3 E S P2 E S 2006 P3 E S 3 P2 E S 3 5 2007 P3 E S P2 E S 2 2008 P3 E S P2 E S P3 E S 1 STRUCTURED QUESTIONS 1. SPM 2003/P2/Q3 Figure 3 shows the flow chart for the industrial manufacture of sulphuric acid and the production of fertilizer Z. Based on figure 3, answer the following questions. (a) Name the process of manufacturing sulphuric acid. …………………………………………………………………………………………… [1 mark] (b) Name the substance X. …………………………………………………………………………………………… [1 mark] (c) Substance X could react directly with water to form sulphuric acid. Explain why this step is not carried out in the industrial process. …………………………………………………………………………………………… (d) …………………………………………………………………………………………… [1 mark] Write the chemical equation when oleum reacts with water to form sulphuric acid. ………………………………………………………………………………………… [1 mark] 1 E Akhmalazmi86@blogspot.com (e) Form 4 Chapter 9 Name the substance Y and the fertilizer Z. Substance Y: ……………………………………………………………………… Fertilizer Z : ……………………………………………………………………… [2 marks] The combustion of petrol in the engines of vehicles produces sulphur dioxide. This gas when dissolved in rain is corrosive. (f) 2. Write a chemical equation when sulphur dioxide reacts with water. ………………………………………………………………………………………… [1 mark] SPM 2006/P2/Q5 (a) Write the chemical equation for the reaction used in Haber Process for the industrial production of ammonia. (b) ………………………………………………………………………………………… [ 2 marks] Graph 5 shows the percentage of ammonia production from Factory P and its competitor Factory Q. (i) Based on Graph 5, compare the percentage of ammonia produced at the pressure of 200 atm from each factory. …………………………………………………………………………………… [1 mark] (ii) Based on your comparison in 5(b)(i), what is the effect of temperature on the percentage of ammonia produced? …………………………………………………………………………………… [1 mark] (iii) One method of increasing the percentage of ammonia is by increasing the pressure. State one problem that may arise from this method. …………………………………………………………………………………… [1 mark] 2 Akhmalazmi86@blogspot.com (iv) Form 4 Chapter 9 How may the problem in 5(b)(iii) be overcome? …………………………………………………………………………………… [1 mark] (c) Ammonia from the Haber Process can be used to manufacture ammonium nitrate fertilizer, NH4NO3. (i) State one other use of ammonium nitrate. (ii) (iii) …………………………………………………………………………………… [1 mark] Complete the chemical equation below for the preparation of ammonium nitrate fertilizer, NH4NO3. ……………………….. + ………………………….. NH4NO3(s) [ 2 marks] The apparatus below is used to make ammonium nitrate solution. Next to the arrow, draw a diagram of the set up of the apparatus used in the preparation of ammonium nitrate crystals [ 2 marks] 3. SPM 2007/P2/Q2 Diagram 2 shows how ammonium sulphate is produced. (a) Process X and process Y are industrial processes. What are the names of each of these processes? X : …………………………………………………………………………………… 3 Akhmalazmi86@blogspot.com (b) Form 4 Chapter 9 Y : …………………………………………………………………………………… [2 marks] What are the three raw materials needed for process X? 1. …………………………………………………………………………………… 2. …………………………………………………………………………………… (c) 3. …………………………………………………………………………………… [3 marks] (i) Write a balanced chemical equation for reaction P. (ii) …………………………………………………………………………………… [1 mark] Use the answer in 2(c)(i) to determine the number of moles of sulphuric acid and the number of moles of ammonia used to produce 1 mole of ammonium sulphate. Sulphuric acid: …………………………………………………………………… Ammonia (d) : …………………………………………………………………… [3 marks] State one use of ammonium sulphate. ………………………………………………………………………………………… [1 mark] 4. SPM 2008/P2/Q1 Diagram 1 shows the manufacture of sulphuric acid. (a) What is the name of this process? ………………………………………………………………………………………… [1 mark] (b) State the name of catalyst X. ………………………………………………………………………………………… [1 mark] (c) (i) State the name of substance Y. ………………………………………………………………………………… 4 Akhmalazmi86@blogspot.com Form 4 Chapter 9 [1 mark] (ii) Substance Y is formed when sulphur trioxide reacts with concentrated sulphuric acid. Write the chemical equation for this reaction. ………………………………………………………………………………… [2 marks] (d) A waste gas is produced during the manufacture of sulphuric acid. Explain briefly how this gas can cause environmental pollution. ………………………………………………………………………………………… ………………………………………………………………………………………… [2 marks] (e) The sulphuric acid produced can be used to manufacture fertilizers. (i) Name one fertilizer manufactured from sulphuric acid. (ii) ………………………………………………………………………………… [1 mark] State another use of sulphuric acid. ………………………………………………………………………………… [1 mark] 5 Akhmalazmi86@blogspot.com Form 4 Chapter 9 Question 1 2003/P2/Q3 (a) Contact Process (b) Sulphur trioxide (c) The reaction gives off a lot of heat. (or Large cloud of sulphuric acid formed) (d) H2S2O7 + H2O 2H2SO4 (e) Substance Y: ammonia Fertilizer Z : ammonium sulphate (f) 2SO2 + O2 + 2H2O 2H2SO4 or SO2 + H2O H2SO3 Question 2 2006/P2/Q5 (a) N2 + 3H2 2NH3 Percentage of ammonia produced from factory Q is higher than factory P. (b) (i) (c) (ii) (iii) (iv) (i) (ii) (iii) At a lower temperature, percentage of ammonia produced is higher. The pipes might explode or break. By using tanks or pipes that can withstand high pressure Use as explosives or cold pack NH3 + HNO3 NH4NO3(s) Question 3 2007/P2/Q2 (a) X : Contact process Y : Haber process (b) 1. Sulphur 2. Air (or oxygen) 3. Water (c) (i) H2SO4 + 2NH3 (NH4)2SO4 (ii) Sulphuric acid: 1 mole Ammonia : 2 moles (d) Ammonium sulphate is use as fertilizer Question 4 2008/P2/Q1 (a) Contact process (b) Vanadium(V) oxide (or vanadium pentoxide) (c) (i) Oleum (ii) SO3 + H2SO4 H2S2O7 (d) Waste gas dissolve in rain water and produces acid rain (or waste gas react with water vapour in the air and produces acid rain.) (e) (i) Ammonium sulphate (or potassium sulphate) [For other accepted answers, please refer to the text book page 152] (ii) Use as electrolyte in car batteries (lead-acid accumulators) or making of detergent.. [For other accepted answers, please to the text book page 152] 6 Akhmalazmi86@blogspot.com Form 4 Chapter 9 FORM 4 CHAPTER 9 MANUFACTURED SUBSTANCES IN INDUSTRY ANALYSIS OF PAST YEAR QUESTIONS FROM 2003 – 2008 Year Paper No. Type of question Question No 5. 2003 P2 S 3 2004 P3 E S P2 E 3 S 2005 P3 E S P2 E S 2006 P3 E S 3 P2 E S 3 5 2007 P3 E S P2 E S 2008 P3 E S 2 P2 E S P3 E S 1 SPM 2004/P2/Q3 (a) Figure 3.1 shows an industrial preparation of sulphuric acid by the Contact Process. Write the chemical equations for the reactions in Stages II and IV. [2 marks] (b) Figure 3.2 shows the waste product from a factory which affects the quality of the environment. Based on Figure 3.2, describe how the waste product affects the quality of the environment. Your description should include the following aspects: Source Process Effect [8 marks] (c) Sarah could easily bend her bangle which is made of pure metal but she could not bend her mother’s bangle which is made of its alloy. By using one suitable example, describe a laboratory experiment to show the hardness of the alloy compared to its pure metal. Explain the difference in hardness of the metal and its alloy in terms of atomic arrangement. [10 marks] 7 E Akhmalazmi86@blogspot.com 6. Form 4 Chapter 9 SPM 2003/P3/Q3 If the body of a car is made of iron, it would easily rust. This is because the iron surface is exposed to air and water. It is also easily dented in an accident. Thus, to reduce these problems the body of the car is made of steel. Referring to the above situation, design a laboratory experiment to compare iron and steel based on one of the following properties: Hardness or rust resistance In designing your experiment it must include the following items: (i) Problem statement (ii) Hypothesis (iii) List of substances and apparatus (iv) Procedure (v) Tabulation of data [17 marks] 7. SPM 2005/P3/Q3 Choose one of the following tasks: Task 1 The copper wire in an electric cable can be easily bent by hand. A one-cent made of an alloy of copper with tin and zinc cannot be bent easily. Referring to the situation above, plan a laboratory experiment to investigate the effect of alloy formation on the hardness of a metal. [For answer to this task, please refer to F5 Chapter 1 Rate of Reaction] Task 2 Buildings in industrial areas are more corroded than those in housing areas. This is because the concentration of acid in rain water is higher in industrial areas. Referring to the situation above, plan a laboratory experiment to investigate the effect of concentration on the rate of reaction between a named acid and a named metal. Your planning must include the following items: (i) Statement of the problem (ii) All the variables (iii) Lists of substances and apparatus (iv) Procedure (v) Tabulation of data [17 marks] 8 Akhmalazmi86@blogspot.com Form 4 Chapter 9 FORM 4 CHAPTER 9 MANUFACTURED SUBSTANCES IN INDUSTRY Question 1 2004/P2/Section C Q3 (a) Stage II : 2SO2 + O2 2SO2 Stage IV : H2S2O7 + H2O 2H2SO4 (b) [Please refer to the pictorial diagram in the text book. page 155 for a more detailed answer] Source: Sulphur dioxide gas (or toxic waste) is produced in the factories (or due to the burning of sulphur in the factories). Process: Sulphur dioxide dissolves in the rain water to form acid rain. Rain water (or toxic waste) from the factories were channeled into rivers. Effect: 1. Acid rain reduces the pH of the soil (or toxic waste poison the soil). 2. Consequently the nutrients in the soils are destroyed. 3. Plants, roots of tree and fishes in the rivers die. 4. Acid rain and acidic river causes corrosion of buildings and bridges. 5. Quality of air decreases. (c) Apparatus: one kilogram weight, metre rule, retort stand and clamp Materials: copper block, bronze block, cellophane tape, thread, steel ball. Procedure 1. A steel ball is stuck onto a copper block by cellophane tape. 2. A weight is hung at a height of 100 cm above the steel ball. 3. The weight is dropped from 100 cm. 4. A dent is formed on the cooper block and its diameter is measured and recorded. 5. Step 1 to step 4 is repeated by replacing the copper block with the bronze block. Observation : It is found that the diameter of the dent in the copper block is larger than that of bronze. [Explaining the difference in hardness in terms of atomic arrangement.] 1. The arrangement of atoms in the copper block is uniform (or orderly) 2. The orderly arrangement of atoms in pure metals enables the layers of atoms to slide on one another when a force is applied. 3. Tin atom which is of different size disturb the orderly arrangement. 4. Hence this reduces sliding between layers of copper atoms. Question 2 2003/P3/Q3 Answer on laboratory experiment based on the property hardness (i) Problem statement Is steel harder than iron? 9 Akhmalazmi86@blogspot.com Form 4 Chapter 9 (ii) Hypothesis Steel is harder than iron. (or Dent formed in steel has a smaller diameter while dent formed on iron has a bigger diameter.) (iii) List of substances and apparatus Iron block, steel block, cellophane tape, thread, one kilogram weight, steel ball bearing, meter rule, retort stand and clamp. (iv) Procedure Please refer to Form 4 Chemistry Practical Book page 148 for the procedure and diagram Note: A two dimensional diagram should be drawn (not a 3-D diagram) Replace the words ‘copper’ and ‘bronze’ (in the practical text book) with the words ‘iron’ and ‘steel’ respectively. (v) Tabulation of data Please refer to Form 4 Chemistry Practical Book page 149 for the data table Answer on laboratory experiment based on the property rust resistant (i) Problem statement Does iron rusts more easily than steel? (ii) Hypothesis Iron has a higher rate of rusting compared to that of steel. (or Iron is less resistance to rust while steel has a higher resistance to rust.) (iii) List of substances and apparatus Iron nail, steel nail, jelly solution, potassium hexacynoferrate(III) solution, sandpaper, test tubes. (iv) Procedure Please refer to Form 4 Chemistry Practical Book page 151 for the procedure and diagram Note: In the practical text book, three types of iron nails were suggested to be used in the experiment. In this question, we need only to use iron nail and steel nail. Hence to answer this question, modify the text book procedure by ignoring the use of stainless nail. (v) Tabulation of data Please refer to Form 4 Chemistry Practical Book page 151 for the data table Question 3 2005/P3/Q3 10 Akhmalazmi86@blogspot.com Form 4 Chapter 9 [Answer for Task 1] (i) Statement of the problem Are alloys harder than its pure metal? (Is alloying of metal increases its hardness?) (ii) All the variables Manipulated variable: Metal and its metal alloy (or copper and bronze; or iron and steel) Responding variable: Diameter of dent Fixed variable : Mass of weight, (or height of the weight, type of ball bearing) (iii) (iv) Lists of substances and apparatus Copper block, bronze block, cellophane tape, thread, one kilogram weight, steel ball bearing, metre rule, retort stand and clamp. Procedure 1. 2. 3. 4. 5. 6. (v) A steel ball is stuck onto a copper block by cellophane tape. A weight is hung at a height of 100 cm above the steel ball. The weight is dropped from 100 cm. A dent is formed on the copper block and its diameter is measured and recorded. The experiment is repeated to obtain more dents and its diameter reading. Step 1 to step 5 is repeated by replacing the copper block with the bronze block. Tabulation of data Metal Copper Bronze Diameter of dent (cm) 11 Average diameter (cm)