PreDent lectue 29Aug
advertisement

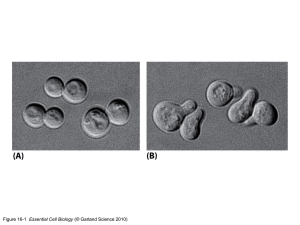
06/08/2014 1. Based on carbon compounds – Organic Chemistry PreDent 2014 2. Depends on chemical reactions occurring in aqueous environment Biological Molecules and Concepts “Chemistry dictates biology” Docent Rachael Sugars Oral Facial Diagnostics and Surgery Dept. Dental Medicine 3. More complex in the cell than any other chemical system 4. Dominated and co-ordinated by complex polymeric molecules Rachael.Sugars@ki.se 5. Tightly regulated Small molecules and macromolecules Small molecules Macromolecules 1 06/08/2014 Polymerisation Key concepts: 1. 2. 3. 4. 5. Importance of carbon Importance of water Importance of selectively permeable membrane Importance of synthesis by polymerisation Importance of self-assembly “The macromolecules that are responsible for most of the form and order characteristic of living systems are generated by the polymerisation of small organic molecules” Becker et al., 2009 Synthesis of biological macromolecules 2 06/08/2014 Self-assembly Figure 2-33 Essential Cell Biology (© Garland Science 2010) Figure 2-32 Essential Cell Biology (© Garland Science 2010) Main families of organic molecules in cells Figure 4-7 Essential Cell Biology (© Garland Science 2010) Figure 2-15 Essential Cell Biology (© Garland Science 2010) 3 06/08/2014 Sugars – Monosaccharide's (aka carbohydrates) e.g. Glucose (CH2O)n n = 3 – 7 C atoms In equilibrium between linear and ring form (e.g. DNA and RNA = pentoses) Central role as an energy form Defined as: Aldosugars – terminal carbonyl Ketosugars – internal carbonyl Condensation reaction Polymers Glycogen in animal cells Starch in plant cells Oligosaccharides Covalent bond = glycosidic bond 2 monomers = disaccharides Between –OH on one sugar and – OH on another Oligosaccharides = trisaccharides, tetrasaccharides etc Water released and bond formed Other biological macromolecules use this process Polysaccharide = 1000’s monosaccharide units Proteins and nucleic acids 4 06/08/2014 Fatty acids Properties Consists of: Saturated 2 hydrophobic hydrocarbon chains (non-polar) Hydrophilic carboxylic acid group (polar) No double bonds between C Pack together tightly Meat and diary Unsaturated Linked to other fatty acids via the carboxylic acid group Termed amphipatic Double bonds produce kinks in chains Prevents close packing Plant oils Figure 2-19 Essential Cell Biology (© Garland Science 2010) Major classes of lipids Formation of membranes Thin sheets enclosing all cells Phospholipids 2 chains Lipid bilayer Figure 2-20 Essential Cell Biology (© Garland Science 2010) 22 5 06/08/2014 Amino acid structure Carboxyl group Amino group Hydrogen atom Side chain (R group) Attached to α-carbon 20 amino acids Proteins Dehydration or condensation 1 C and 1 N directly linked H20 excluded Peptide bond Each addition lengthens the sequence Figure 4-3 Essential Cell Biology (© Garland Science 2010) Figure 4-1 Essential Cell Biology (© Garland Science 2010) 6 06/08/2014 Polypeptide chain Protein organisation Figure 4-2 Essential Cell Biology (© Garland Science 2010) Nucleotides Short term carriers of energy Nucleic acids Figure 2-24 Essential Cell Biology (© Garland Science 2010) 7 06/08/2014 Nucleic Acids DNA vs RNA DNA RNA A=T A=U G=C G=C Double strand Single strand 2’ C has H 2’ C has OH Long Short mRNA tRNA rRNA DNA is made from 4 nucleotide building bocks Figure 5-2 Essential Cell Biology (© Garland Science 2010) Figure 5-6a Essential Cell Biology (© Garland Science 2010) DNA Double helix Anti-parallel strands Hydrogen bonds 8 06/08/2014 Study questions! 9