15
Yeast and Molasses
Yeast and Molasses
Examining the Effect of Food Concentration on
Fermentation
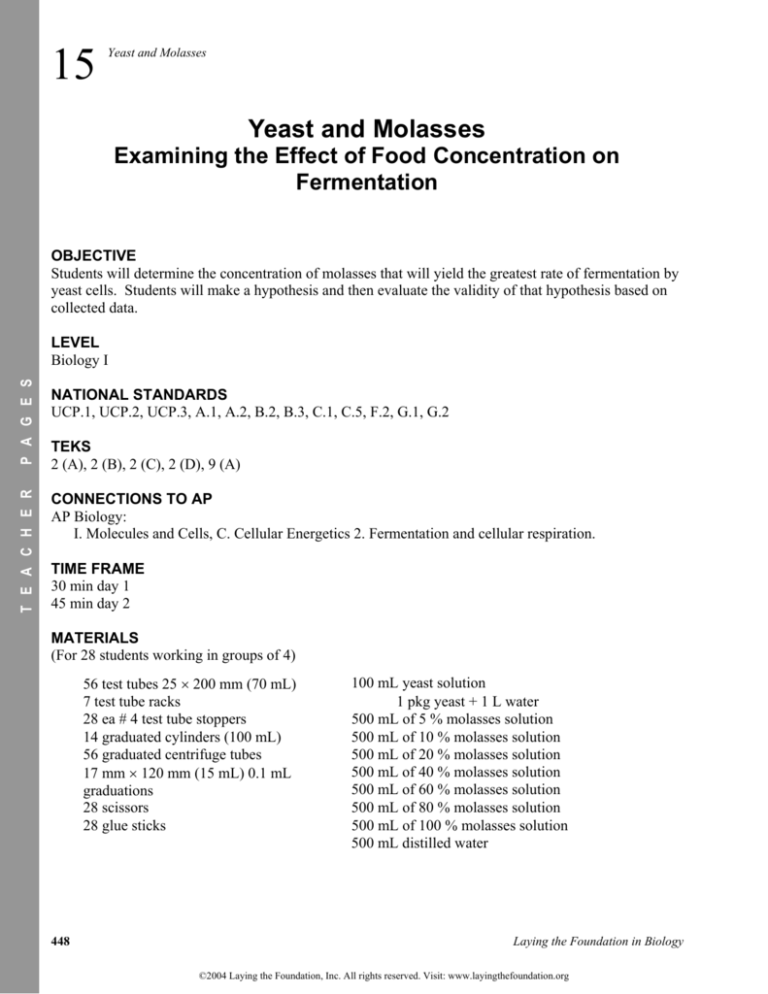
OBJECTIVE
Students will determine the concentration of molasses that will yield the greatest rate of fermentation by
yeast cells. Students will make a hypothesis and then evaluate the validity of that hypothesis based on
collected data.
P A G E S
NATIONAL STANDARDS
UCP.1, UCP.2, UCP.3, A.1, A.2, B.2, B.3, C.1, C.5, F.2, G.1, G.2
T E A C H E R
LEVEL
Biology I
CONNECTIONS TO AP
AP Biology:
I. Molecules and Cells, C. Cellular Energetics 2. Fermentation and cellular respiration.
TEKS
2 (A), 2 (B), 2 (C), 2 (D), 9 (A)
TIME FRAME
30 min day 1
45 min day 2
MATERIALS
(For 28 students working in groups of 4)
56 test tubes 25 200 mm (70 mL)
7 test tube racks
28 ea # 4 test tube stoppers
14 graduated cylinders (100 mL)
56 graduated centrifuge tubes
17 mm 120 mm (15 mL) 0.1 mL
graduations
28 scissors
28 glue sticks
448
100 mL yeast solution
1 pkg yeast + 1 L water
500 mL of 5 % molasses solution
500 mL of 10 % molasses solution
500 mL of 20 % molasses solution
500 mL of 40 % molasses solution
500 mL of 60 % molasses solution
500 mL of 80 % molasses solution
500 mL of 100 % molasses solution
500 mL distilled water
Laying the Foundation in Biology
©2004 Laying the Foundation, Inc. All rights reserved. Visit: www.layingthefoundation.org
Yeast and Molasses
15
TEACHER NOTES
This lab activity supplements a unit on cellular respiration.
Make the following molasses solutions by mixing:
Amount of Molasses
Amount of Water
0%
0 mL
500 mL
5%
25 mL
475 mL
10 %
50 mL
450 mL
20 %
100 mL
400 mL
40 %
200 mL
300 mL
60 %
300 mL
200 mL
80 %
400 mL
100 mL
100 %
500 mL
0 mL
T E A C H E R
Percentage
If time permits, you may want each lab group to make their own molasses solution by mixing the
following:
Amount of Molasses
Amount of Water
0%
0 mL
40 mL
5%
2 mL
38 mL
10 %
4 mL
36 mL
20 %
8 mL
32 mL
40 %
16 mL
24 mL
60 %
24 mL
16 mL
80 %
32 mL
8 mL
100 %
40 mL
0 mL
P A G E S
Percentage
It may take some time to get the molasses and the water thoroughly mixed.
Prepare a stock yeast solution by adding 1 package of brewer’s yeast (7g) to 1 L of warm water about 15
minutes before class. When the yeast is needed, dilute 30 mL of the stock solution with 70 mL of warm
water.
Laying the Foundation in Biology
©2004 Laying the Foundation, Inc. All rights reserved. Visit: www.layingthefoundation.org
449
15
Yeast and Molasses
Part I of this lab investigates the relationship between fermentation and increasing concentration of the
food source, molasses. The rate of fermentation is indicated by the amount of carbon dioxide produced.
Typically, students predict that increasing concentrations of molasses will result in an increased rate of
fermentation. However, the results of this activity will show that when the concentration of molasses is
above 80%, there is a decrease in the amount of energy produced. This decline is attributed to resultant
low levels of water within the cells.
Prepare a transparency of the data table to facilitate the sharing of data.
Part II is a cut and paste exercise which examines the specific events of glycolysis and fermentation in a
manner engaging to tactile/kinesthetic learners. If you have no need to differentiate your instructional
activities you may simply have the students write in the missing information.
T E A C H E R
P A G E S
POSSIBLE ANSWERS TO THE CONCLUSION QUESTIONS AND SAMPLE DATA
DATA AND OBSERVATIONS
Data Table 1
Amount of CO2 Collected (mL)
Test Tube #
1
2
3
4
5
6
7
8
0%
5%
10 %
20 %
40 %
60 %
80 %
100 %
Individual Team
Data
0
5.0
11.0
14.0
15.0
15.0
7.5
0
Team # 1
0
5.5
10.5
15.0
15.0
15.0
7.0
0
Team # 2
0
5.0
10.0
13.5
15.0
150.
7.0
1
Team # 3
0
6.0
10.0
12.5
15.0
15.0
8.0
0
Team # 4
0
4.0
10.5
15.0
15.0
15.0
7.5
0
Team # 5
0
5.5
12.0
15.0
15.0
15.0
7.0
0
Team # 6
0
4.0
10.5
15.0
14.5
15.0
8.0
0
Team # 7
0
5.5
11.0
14.0
15.0
13.0
7.5
0
0.0
5.1
10.6
14.4
14.9
14.7
7.4
0.1
Percent of
Molasses
Class Average
CONCLUSION QUESTIONS
1. What happened to the amount of carbon dioxide gas produced as the concentration of molasses
increased?
In the beginning, an increase in the concentration of molasses resulted in an increase in the
production of carbon dioxide, however, after a certain point, an increase in the concentration of
450
Laying the Foundation in Biology
©2004 Laying the Foundation, Inc. All rights reserved. Visit: www.layingthefoundation.org
Yeast and Molasses
15
molasses resulted in a decrease of carbon dioxide production. This is because there is insufficient
water to support life for the yeast cells.
2. What percentage of molasses resulted in the greatest amount of fermentation? Does this result
support your hypothesis?
The individual data indicated that the greatest amount of fermentation occurred when the
molasses was concentrated between twenty and sixty percent. The data supported the hypothesis
up to sixty percent and then after that, an increase in the concentration of molasses resulted in a
decrease in the amount of fermentation produced. This is because the molasses solution becomes
hypertonic to the yeast cells, and the yeast cells are dehydrating.
T E A C H E R
3. Design an experiment based on this protocol that would investigate the effect of temperature on
fermentation.
This is an open-ended question that will result in a variety of answers but the following is a
possible answer: The data indicated that a molasses concentration of 20 60% resulted in the
greatest amount of energy production. This lab could use the same protocol with 40% molasses
for three sets of test tubes. One set could be put into the refrigerator, the second in an incubator,
and the third at room temperature for 24 hours. Require the students to include control in their
experimental design.
P A G E S
Laying the Foundation in Biology
©2004 Laying the Foundation, Inc. All rights reserved. Visit: www.layingthefoundation.org
451
15
Yeast and Molasses
PART II
Diagram Key — Biochemical Pathway for Glycolysis and Fermentation.
CH2 OH
H
C
HO
C
H
O
OH
H
H
C
OH
C
C
H Glucose OH
ATP
P A G E S
CH2 O– P
H
C
T E A C H E R
HO
C
H
O
OH
H
C
H
C
OH
ADP
H
C
OH
Glucose 6-phosphate
CH2 O– P
O
2. Atoms are rearranged and
glucose 6-phosphate is turned into
fructose 6-phosphate.
CH2 OH
C
H
1. A phosphate is added to glucose. It
comes from ATP. This phosphate
increases the amount of energy of
glucose.
C
H
HO
OH
C
C
HO Fructose H
16- bisphosphate
452
Laying the Foundation in Biology
©2004 Laying the Foundation, Inc. All rights reserved. Visit: www.layingthefoundation.org
Yeast and Molasses
CH2 O– P
O
CH2 OH
C
H
C
H
HO
C
HO
C
H
OH
ATP
Fructose 6-phosphate
CH2 O– P
O
ADP
3. Another phosphate is added to
fructose 6-phosphate. It comes from
ATP. This phosphate increases the
amount of energy of fructose.
CH2 O– P
C
C
H
HO
OH
C
C
HO Fructose H
T E A C H E R
H
15
4. Fructose 1-6 bisphosphate has so
much energy and is so unstable that this
hexose is cleaved into two trioses,
phosphoglyceralaldehyde or PGAL and
dihydroxacteone phosphate.
1-6 bisphosphate
H
C =O
C =O
CHOH
CH2 O– P
CH2 O– P
Dihydroxyacetone
phosphate
Phosphoglyceraldehyde
5. Atoms are rearranged and dihydroxyacetone phosphate is turned into
phosphoglyceraldehyde, PGAL. From
This point in time, everything is multiplied
by a factor of two because there are two
trioses.
Laying the Foundation in Biology
©2004 Laying the Foundation, Inc. All rights reserved. Visit: www.layingthefoundation.org
P A G E S
CH2 OH
453
T E A C H E R
P A G E S
15
Yeast and Molasses
454
Laying the Foundation in Biology
©2004 Laying the Foundation, Inc. All rights reserved. Visit: www.layingthefoundation.org
Yeast and Molasses
15
T E A C H E R
P A G E S
Laying the Foundation in Biology
©2004 Laying the Foundation, Inc. All rights reserved. Visit: www.layingthefoundation.org
455
T E A C H E R
P A G E S
15
Yeast and Molasses
REFERENCES
Biological Science, Interaction of Experiments and Ideas. Englewood Cliffs: Prentice-Hall, Inc., 1983.
pp. 16 22
456
Laying the Foundation in Biology
©2004 Laying the Foundation, Inc. All rights reserved. Visit: www.layingthefoundation.org
Yeast and Molasses
15
Yeast and Molasses
Examining the Effect of Food Concentration on
Fermentation
All cells need energy, and the most useful energy molecule for cells is ATP. The full name given to
ATP by chemists is adenosine triphosphate. ATP is composed of the sugar, ribose, and the nitrogenous
base, adenine, and three phosphate groups. Two of the bonds connecting the phosphates are considered
high-energy bonds and, when broken, useful energy is released. Cells use this energy to power a variety
of cellular activities. Cells produce ATP through the process of respiration. During respiration, energy
is moved from the bonds of organic compounds into the phosphate bonds of ATP. If a phosphate group
is removed from ATP, a molecule called adenosine diphosphate (ADP) will be formed. ADP has one
less phosphate group than ATP and thus less energy. If two phosphate groups are removed from ATP, a
molecule called adenosine monophosphate (AMP) is formed.
Adenine
H
C
N
C
HC
C
N
CH
N
Energy-rich bonds
N
C
H
O-
H
O
C
C
H
C
H
C
OH
OH
H
O
O
P
O
O-
OP
O
O
P
OH
O
H
3 Phosphates
Ribose
Many biochemical reactions require the addition of phosphates to one or more reactants in order to
proceed. The addition of these phosphates increases the energy content of the molecule. For example,
in the biochemical reaction called glycolysis, the process of breaking down glucose begins by adding a
phosphate to the molecule producing glucose-phosphate. Glucose-phosphate has more energy than plain
glucose because of the addition of a phosphate group.
Cells need a plentiful supply of ATP. Once the ATP is used and converted into ADP, the cells need to
regenerate ATP by adding a phosphate to ADP. Since energy was released during the breaking of the
phosphate bond, the reverse process of forming a phosphate bond requires an energy input.
Laying the Foundation in Biology
©2004 Laying the Foundation, Inc. All rights reserved. Visit: www.layingthefoundation.org
457
15
Yeast and Molasses
The energy needed to regenerate ATP is transferred by cells from the organic compounds in food
sources such as glucose, sucrose, or other such organic compounds. Glucose has considerably more
energy than ATP. One molecule of ATP has approximately 16 kcal/mole whereas glucose has
approximately 680 kcal/mole. You may ask, why not use glucose instead of ATP as the cell energy
currency? Cells cannot directly use glucose as an energy source because glucose has too much energy.
Releasing all of glucose’s energy at once would be like putting a match into a gas can. The cell cannot
handle the release of such large amounts energy at once. The resulting increase in temperature would
denature the enzymes and destroy the cell. Instead, cell respiration releases the energy from organic
compounds in small amounts at a time through a series of steps. Another analogy that illustrates using
glucose as a direct energy source in the cell would be like you trying to buy a candy bar with a one
thousand dollar bill. You have money but it is in an unusable form. The bill has to be taken to the bank
and exchanged for useable money such as ten-dollar bills. In the cell’s energy economy, glucose is like
the one thousand dollar bill and ATP is like ten-dollar bills. The cell can use ATP directly and easily.
The process by which cells retrieve the energy from molecules such as glucose is called cellular
respiration. The chemical equation below summarizes the process of cellular respiration.
There are three major parts to cell respiration. The three parts to cellular respiration are glycolysis, the
Krebs cycle, and oxidative phosphorylation. Glycolysis occurs in the cytoplasm of the cell. The major
events in the process of glycolysis are the following:
During the process of glycolysis no oxygen is required.
The other 34 molecules of ATP are made in the Krebs cycle and oxidative phosphorylation. These
processes occur in the inner compartment of the mitochondria. It is at the very end of these processes
that oxygen is needed. Respiration is often referred to as aerobic respiration because oxygen is required.
If there is no oxygen present, then the Krebs cycle and oxidative phosphorylation will not occur.
If oxygen is not present, then glycolysis can continue to make pyruvic acid and two molecules of ATP.
After all, the synthesis of two ATP molecules is better than making none. The limiting factor in this
process is having enough NAD to make the NADH. In order to regenerate NAD, several additional
steps are needed. These additional steps complete the process known as fermentation. There are several
types of fermentation. The most common types of fermentation are lactic acid fermentation and alcohol
fermentation. Muscle cells perform lactic acid fermentation when muscles are vigorously contracting
and are unable to obtain enough oxygen. Yeast cells, plant cells and certain bacteria perform alcoholic
458
Laying the Foundation in Biology
©2004 Laying the Foundation, Inc. All rights reserved. Visit: www.layingthefoundation.org
Yeast and Molasses
15
fermentation in the absence of oxygen. The alcohol produced is ethanol and during this reaction carbon
dioxide is released.
This lab exercise uses yeast cells to investigate the relationship between fermentation and food
concentrations. If yeast cells have more food available, will they generate more ATP? Yeast cells use
food sources like molasses through alcoholic fermentation to regenerate ATP. During alcoholic
fermentation, carbon dioxide is released as a by-product. This lab measures the amount of carbon
dioxide released as an indicator of the amount of fermentation occurring. Below are the final steps
added to glycolysis to complete the process of fermentation.
PURPOSE
In this activity you will investigate the process of fermentation and its relationship to the availability of
food for fermentation.
Laying the Foundation in Biology
©2004 Laying the Foundation, Inc. All rights reserved. Visit: www.layingthefoundation.org
459
15
Yeast and Molasses
MATERIALS
Part I
8 test tubes 25 200 mm (70 mL)
test tube rack
4 ea # 4 test tube stoppers
graduated cylinders (100 mL)
8 graduated centrifuge tubes
17 mm 120 mm (0.1 mL graduations)
Part II
scissors
yeast solution
5 % molasses solution
10 % molasses solution
20 % molasses solution
40 % molasses solution
60 % molasses solution
80 % molasses solution
100 % molasses solution
distilled water
glue stick
PROCEDURE
PART I
1. Formulate a hypothesis that predicts the relationship between the amount of fermentation and an
increasing amount of available food. Record your hypothesis on your student answer page. Your
teacher will divide the class into groups of 3 4 students. Every student should have the opportunity
to participate in the experiment.
2. Obtain the above materials and label the large test tubes 1 8.
3. Add 40 mL of the indicated molasses solution to each of the following test tubes:
# 1 — 0 % molasses/ only distilled water
# 2 — 5 % molasses
# 3 — 10 % molasses
# 4 — 20 % molasses
# 5 — 40 % molasses
# 6 — 60 % molasses
# 7 — 80 % molasses
# 8 — 100 % molasses
4. Add 10 mL of yeast solution to each test tube.
5. Stopper each tube and shake to mix thoroughly.
6. Remove the stopper and rinse the stopper with water.
460
Laying the Foundation in Biology
©2004 Laying the Foundation, Inc. All rights reserved. Visit: www.layingthefoundation.org
Yeast and Molasses
15
7. Obtain a small centrifuge tube and notice that it has markings on the sides of the tube in mL. The
small tube needs to be filled with the yeast-molasses solution. Do this by inverting the small
centrifuge tube and sliding it into the large test tube. Then, put the large stopper into the large tube
and hold it on its side. When the small tube is completely filled with the suspension, slowly move
the large tube back to its upright position. If there is any air bubble in the small tube, repeat the
procedure until no air is present.
8. Allow the tubes to sit for 24 hours. After 24 hours, measure the gas in the centrifuge tube for each
tube by observing the amount of gas in the tube using the gradations on the side of the centrifuge
tube. Record your data in Data Table 1 on your student answer page. Collect data for each group in
the classroom and average the data.
9. Graph your data and the class-averaged data.
Optional — These calculations can be done on a TI-83 calculator by doing the following:
1. To make a data table press
, the select EDIT (Figure 1) and press
are columns or lists to record data.
Laying the Foundation in Biology
©2004 Laying the Foundation, Inc. All rights reserved. Visit: www.layingthefoundation.org
. Notice that there
461
15
Yeast and Molasses
Figure 1
Figure 2
2. To clear a list that might have data in it, put the cursor at the very top of the list so that the name of
the column is highlighted. Press
followed by the
.
3. Now enter the percent of molasses in L1 starting at 0 for the first entry and ending at 100 percent for
the last entry. In L2, record the amount of carbon dioxide collected. In L3, record the class average
for the amount of carbon dioxide collected. (Figure 2). To view this graphically, press
,
. At this time make sure to put your cursor on PLOT 1 and press
move the cursor down to ON and press
4. Highlight the Xlist and press
,
[L1].
5. Highlight the Ylist and press
,
[L2].
6. Highlight the box symbol for the Mark and press
Figure 3
. Then
. Both PLOT 1 and ON should be highlighted. All
other plots should be inactivated. Highlight the line graph (Figure 3) and press
462
,
.
(Figure 3).
Figure 4
Laying the Foundation in Biology
©2004 Laying the Foundation, Inc. All rights reserved. Visit: www.layingthefoundation.org
Yeast and Molasses
7. To put a second line on the graph, position the cursor on Plot 2 and press
down to ON and press
graphs (Figure 4) and press
15
. Move the cursor
. Both PLOT 2 and ON should be highlighted. Highlight the line
.
8. Highlight the Xlist and press
,
[L1].
9. Highlight the Ylist and press
,
[L3].
(Figure 4).
10. Highlight the cross hairs symbol for the Mark and press
11. Press,
then press
. A graph should appear that will have automatically adjusted the
axes so that they fit the window. (Figures 5 and 6).
Figure 5
Figure 6
It may appear that there is only one line on the graph if your data is close to the class average. To
and then use the
and
to
demonstrate that both lines are present press
differentiate between the points. Look at the figures above. In Figure 5, the data point, Y=14.9, is
class averaged data and in figure 6, the data point Y=15, is the student’s data.
PART II
In this portion of the activity you will practice sequencing the events of glycolysis and fermentation. As
you read about the steps of these processes, look at the accompanying diagram on your student answer
page. You will notice that there are names of products and descriptions of the reaction missing. The
empty boxes indicate missing items. At the end of the diagram is a list of missing parts. Using scissors
and a glue stick, fill in the missing items to make the biochemical pathway complete. Be sure to place
either the missing step or molecule in the correct sequence.
1. A phosphate is added to glucose. It comes from ATP. This phosphate increases the amount of
energy of glucose.
Laying the Foundation in Biology
©2004 Laying the Foundation, Inc. All rights reserved. Visit: www.layingthefoundation.org
463
15
Yeast and Molasses
2. Atoms are rearranged and glucose 6-phosphate is turned into fructose 6-phosphate.
3. Another phosphate is added to fructose 6-phosphate. It comes from ATP. This phosphate increases
the amount of energy of fructose.
4. The increased energy and phosphate makes fructose 1,6-biphosphate causing it to split into two
molecules, phosphoglyceraldehyde (PGAL) and dihydroxyacetone phosphate.
5. Atoms are rearranged to convert dihydroxyacetone phosphate into PGAL. Each PGAL will continue
through the process. For this reason there is a “2” in front of the product names in the remaining
steps.
6. Hydrogens are stripped from each phosphoglyceraldehyde and transferred to NAD+. In addition, an
inorganic phosphate group is added to the molecule. (The phosphate group comes from the
cytoplasm.) This step produces 1, 3-biphosphoglyceric acid.
7. Each 1, 3- biphosphoglyceric acid molecule gives up a phosphate to ADP forming ATP. This step
produces 3-phosphoglyceric acid.
8. The phosphate group found on carbon number three is transferred to carbon number two producing
2-phosphoglyceric acid.
9. A dehydration reaction occurs as a water molecule is removed to form phosphoenolpyruvic acid or
PEP.
10. Phosphoenolpyruvic acid transfers its phosphate to ADP to form ATP. This produces pyruvic acid.
If oxygen is present then the pyruvic acid is used in the Krebs cycle. If NO oxygen is present, then
fermentation occurs.
11. Lactic acid fermentation- Hydrogens are transferred to pyruvic acid from NADH to form lactic acid
and NAD+. Alcoholic fermentation- Hydrogens are transferred to pyruvic acid from NADH to form
lactic acid and NAD+ and a molecule of carbon dioxide is produced.
464
Laying the Foundation in Biology
©2004 Laying the Foundation, Inc. All rights reserved. Visit: www.layingthefoundation.org
Yeast and Molasses
15
Name _____________________________________
Period _____________________________________
Yeast and Molasses
Examining the Effect of Food Concentration on
Fermentation
HYPOTHESIS
DATA AND OBSERVATIONS
Data Table 1
Amount of CO2 Collected (mL)
Test Tube #
Percent of
Molasses
1
2
3
4
5
6
7
8
0%
5%
10 %
20 %
40 %
60 %
80 %
100 %
Individual Team
Data
Team # 1
Team # 2
Team # 3
Team # 4
Team # 5
Team # 6
Team # 7
Class Average
Laying the Foundation in Biology
©2004 Laying the Foundation, Inc. All rights reserved. Visit: www.layingthefoundation.org
465
15
Yeast and Molasses
CONCLUSION QUESTIONS
1. What happened to the amount of carbon dioxide gas produced as the amount of molasses increased?
2. What percentage of molasses resulted in the greatest amount of fermentation? Does this result
support your hypothesis? Give a possible explanation for any tube(s) that did not support your
hypothesis.
3. Design an experiment based on this protocol that would investigate the effect of temperature on
fermentation.
466
Laying the Foundation in Biology
©2004 Laying the Foundation, Inc. All rights reserved. Visit: www.layingthefoundation.org
Yeast and Molasses
15
PART II
DIAGRAM OF GLYCOLYSIS & FERMENTATION
CH2 OH
H
C
HO
C
H
O
OH
H
H
C
OH
C
C
H Glucose OH
1. A phosphate is added to glucose. It
comes from ATP. This phosphate
increases the amount of energy of
glucose.
CH2 O– P
H
C
HO
C
H
O
H
OH
H
C
H
C
OH
C
OH
Glucose 6-phosphate
CH2 O– P
O
CH2 OH
C
H
2. Atoms are rearranged and
glucose 6-phosphate is turned into
fructose 6-phosphate.
C
H
HO
C
HO
C
H
OH
Laying the Foundation in Biology
©2004 Laying the Foundation, Inc. All rights reserved. Visit: www.layingthefoundation.org
467
15
Yeast and Molasses
CH2 O– P
O
CH2 OH
C
H
C
H
HO
C
HO
C
H
OH
3. Another phosphate is added to
fructose 6-phosphate. It comes from
ATP. This phosphate increases the
amount of energy of fructose.
Fructose 6-phosphate
CH2 O– P
O
CH2 O– P
C
H
C
H
HO
OH
C
C
HO Fructose H
1-6 bisphosphate
468
CH2 OH
H
C =O
C =O
CHOH
CH2 O– P
CH2 O– P
Dihydroxyacetone
phosphate
Phosphoglyceraldehyde
5. Atoms are rearranged and dihydroxyacetone phosphate is turned into
phosphoglyceraldehyde, PGAL. From
This point in time, everything is multiplied
by a factor of two because there are two
trioses.
Laying the Foundation in Biology
©2004 Laying the Foundation, Inc. All rights reserved. Visit: www.layingthefoundation.org
Yeast and Molasses
Laying the Foundation in Biology
©2004 Laying the Foundation, Inc. All rights reserved. Visit: www.layingthefoundation.org
15
469
15
470
Yeast and Molasses
Laying the Foundation in Biology
©2004 Laying the Foundation, Inc. All rights reserved. Visit: www.layingthefoundation.org
Yeast and Molasses
Laying the Foundation in Biology
©2004 Laying the Foundation, Inc. All rights reserved. Visit: www.layingthefoundation.org
15
471
15
Yeast and Molasses
Here are the missing items to make glycolysis and fermentation complete:
472
Laying the Foundation in Biology
©2004 Laying the Foundation, Inc. All rights reserved. Visit: www.layingthefoundation.org
Yeast and Molasses
Laying the Foundation in Biology
©2004 Laying the Foundation, Inc. All rights reserved. Visit: www.layingthefoundation.org
15
473