Moles Worksheet Key
advertisement

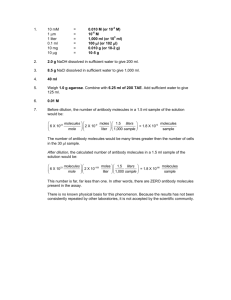
0.1. WORKSHEET – MOLES AND MASSES: KEY Name: __________________________________ Background information: At 0 °C and atmospheric pressure, the number density of any gas is n = 2.70 × 1019 atoms (or molecules ) cm3 A. 24.0 L of gas made up of some type of molecule is held at 0 °C and atmospheric pressure. A1. What is the volume of this gas in cm3? ⎛ 1cm3 ⎞ ⎛ 1000 mL ⎞ 4 3 24.0 L ⎜ ⎟⎜ ⎟ = 2.40 ×10 cm 1mL 1 L ⎝ ⎠⎝ ⎠ A2. How many gas molecules are in this volume? The number of gas molecules, N, is the number density times the volume: N = nV ⎛ 2.70×1019 molecules ⎞ 4 3 =⎜ ⎟ ( 2.40×10 cm ) 3 cm ⎝ ⎠ 23 =6.48×10 molecules A3. What is the number of moles of gas molecules in this volume? nM = N NA ⎛ ⎞ 1mole = 6.48 ×1023 molecules ⎜ ⎟ 23 ⎝ 6.022×10 molecules ⎠ =1.08 moles A4. What is the molecular weight of the gas? B. There are 3.20 moles of Cl2 molecules in 4.00 L volume B1. How many molecules are present in one mole of this gas? NA B2. How many molecules are present in volume of 4.00 L? N = nM N A molecules ⎞ ⎛ = ( 3.20 moles ) ⎜ 6.02 ×1023 ⎟ mole ⎠ ⎝ 1.93×1024 molecules B3. What is the number density expressed in units of n = 4.82 ×1023 = 4.82 ×1020 molecules molecules and ? L cm3 molecules ⎛ 1 L ⎞ ⎛ 1mL ⎞ ⎜ ⎟⎜ 3 ⎟ L ⎝ 1000mL ⎠ ⎝ 1cm ⎠ molecules cm3 C. Stearic acid is described by the chemical formula C17H35COOH. C1. What is the molecular weight of stearic acid? Add the masses of each individual component. Check SFS element number atomic weight H 36 1.00794 C 18 12.011 O 2 15.9994 total weight TOTAL 36.2858 216.198 31.9988 284.4826 This says that the molecular weight of stearic acid is 284.483 u C2. What the mass of 1.00 mole of stearic acid? 284.483 g C3. How many molecules are in 1.00 gram of stearic acid? There are N A molecules in 284.482 g, so 1 g ⎛⎜ 6.02 × 1022 molecules ⎞⎟ ⎟⎟ = 2.12 x 1020 molcules ⎜ ⎜⎝ 284.483 g ⎠⎟ C4. How many atoms are there in 1.00 cm3? You would need to know a density to calculate this. There is no set volume for a mole of a solid as there is for an ideal gas at STP.