Origin and Differentiation of Magma
advertisement

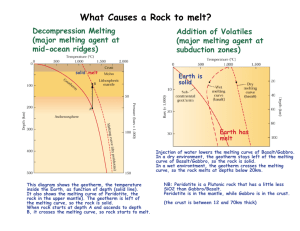
Origin and Differentiation of Magma see Hefferan & O'Brien (2010) sections 8.1-8.4 Origin of Magma partial melting: If rock is completely melted, the melt composition would be the same as the elemental composition of the rock. However, the Earth's interior is not hot enough to completely melt rock in the mantle and crust today. In certain tectonic environments in the interior, rock can undergo anatexis, partial melting in which the lower melting temperature components in the rock melt, leaving behind the more refractory, higher melting temperature components. Partial melting is subliquidus melting - melting at temperatures above the solidus but below the liquidus. In equilibrium melting, the melt remains in contact with the un-melted material (the restite) and ions can diffuse in and out of the crystals to maintain chemical equilibrium between the melt and the solid as melting proceeds and the degree of melting proceeds. in fractional or disequilibrium melting, early-formed melts move away from the parent material/restite so that later melts are compositionally distinct. The Earth's interior is very hot and temperature increases with depth, but so too does pressure. And as pressure increases, so does the temperature required for melting. So despite the fact the temperatures deep in the mantle exceed the melting temperature of rock at the Earth’s surface, the high temperatures are still below the melting temperature for mantle rocks except in certain tectonic environments. We know that the crust and mantle are solid from the fact that shear waves from earthquakes travel through the entire crust and mantle (shear wave, S waves, don’t travel through liquids). What could allow rock in the Earth's interior to melt? raise its temperature: Small amounts of magma can be produced around high temperature mafic intrusions. Rock can also be heated along major shear zones, e.g., in continental collisions to produce small volumes of low-temperature, felsic magma. decrease its pressure – decompression melting: Where mantle rock is rising beneath midocean ridges, continental rifts, and hotspots the reduction in pressure brings rock near its melting temperature, resulting in partial melting of the ultramafic mantle rock to produce mafic magma. add volatiles – flux melting: In subduction zones, at depths between ~80-125 km, sinking ocean crust undergoes metamorphic dewatering. Hydrous minerals like amphiboles and serpentine (phyllosilicate) metamorphose into denser, non-hydrous minerals thereby releasing water. This water permeates the mantle rock above the subducting slab. The water depresses the solidus temperature allowing partial melting of ultramafic mantle peridotite to produce mafic magma. partial melting (anatexis), melting of the lowest melting temperature components, produces magma that is less mafic than the parent rock partial melting of ultramafic mantle peridotite produces mafic magma removes the lowest melting temperature components e.g., melts Fe-rich olivine and Na-rich plagioclase leaves behind the highest melting temp components e.g., Mg-rich olivine and Ca-rich plagioclase the melt is enriched in SiO2, Na, & K the residual solid (restite) is enriched in Mg & Ca Minor & Trace Elements incompatible elements elements that don’t fit well into common crystal lattice sites (too big or wrong charge) prefer to go into a melt during partial melting - concentrated in the resulting magma - depleted in the residual rock left behind incompatible elements include: Large Ion Lithopile (LIL) elements (large for their charge) Cs, Ba, Rb, Sr, U, Pb K, Zr, Th, Ta Light Rare Earth elements (LREE) REEs La through Gd compatible elements other elements are very stably bonded in silicate structures they are highly immobile and remain in the restite, depleted in the magma compatible elements include: High Field Strength (HFS) elements (small for their charge) Ti, Ni, Cr, V, Zr, Hf, Nb, Ta, Y Heavy Rare Earth Elements (HREE) small percent melts result in magma greatly enriched in incompatible elements large percent melts result in less distinction between partial melt and parent How do we know if an element is enriched or depleted in a magma or restite? Compare the concentration of the element in the rock in question to that found in chondritic meteorites. Chondritic meteorites are believed to have the non-volatile composition of the solar nebula, and the bulk silicate composition of Earth before differentiation of the crust by partial melting of mantle rocks Petrologists and geochemists studying crustal rocks crystalized from mantle-derived magmas describe the mantle parent rocks for those magmas as either having been depleted mantle rock or enriched or primitive mantle rock. Modification of Primary (mafic) Magma Bowen (1928) noticed that in the Palisade Sill intrusion heavy olivine crystals seemed to have sunk to the bottom while lighter plagioclase were more concentrated near top fractional crystallization (Bowen believed) separation of early formed crystals causes magma composition to change become more felsic and change elemental balances (it does, but he also believed that fractional crystallization could produce all of the range of mafic through felsic magmas from a parent mafic magma produced in the mantle which is now not believed to be true) Bowen’s reaction series - order of crystallization the most ionic minerals crystallize at highest temperature olivine, then pyroxenes, then amphiboles, then biotite same time as Ca-rich plag, then intermediate plag, then sodium rich plag kspar, +/- muscovite, quartz crystallize at lowest temps Continuous Reaction Series Ca plag. to Na plag. - solid solution series - all the same structure composition progressively changes as magma comp. becomes more sodic Discontinuous Reaction Series Olivine-Pyroxene-Amphibole-Biotite - each is a different xtal structure under equilibrium xtalization olivine changes to pyroxene pyroxene to amphibole, amphibole to biotite as magma becomes more silicic a reaction rim occurs if non-equilibrium pyroxene surrounding olivine or zoned feldspar - Na-rich surrounding Ca-rich plagioclase mechanisms of fractional crystallization: gravitational separation: early-formed heavy crystals sink to bottom of magma chamber marginal accretion: early-forming minerals form on cool wall of magma chamber ilter pressing crystals left behind as migrating magma squeezes through fractures assimilation of country rocks: magma chemistry changes as a result of reaction with country rocks or melting of lower melting point country rock into the magma magma mingling: sometimes different batches of independently differentiated magmas will mingle as they rise into the crust producing a hybrid or they may form a zonation of different chemical variations if not well mixed xenoliths: foreign rock within an igneous rock body are evidence for assimilation and magma mingling