atomic weights of the elements
advertisement

Pure & Appi. Chem.., Vol.52, pp.2349—2384.
Pergamon Press Ltd. 1980. Printed in Great Britain.
© IUPAC
0033—4545/80/1001—2349$02.00/0
INTERNATIONAL UNION OF PURE
AND APPLIED CHEMISTRY
INORGANIC CHEMISTRY DIVISION
COMMISSION ON ATOMIC WEIGHTS AND
ISOTOPIC ABUNDANCES*
ATOMIC WEIGHTS OF THE ELEMENTS
1979
Prepared for publication by
N. E. HOLDEN
Brookhaven National Laboratory, Upton, New York 11973, USA
*Membershjp of the Commission for the period 1979 -
81
is as follows:
Chairman: N. E. HOLDEN (USA); Secretary: R. L. MARTIN (Australia); Titular Members:
R. C. BARBER (Canada); I. L. BARNES (USA); P. J. DE BIEVRE (Belgium); R.
HAGEMANN (France); W. H. JOHNSON (USA); T. J. MURPHY (USA); Associate
Members: A. E. CAMERON (USA); S. FUJIWARA (Japan); R. GONFIANTINI (Italy); N.
N. GREENWOOD (UK); Y. HORIBE (Japan); J. R. DE LAETER (Australia); H. S. PEISER
(USA); National Representative: V. I. GOLDANSKII (USSR).
ATOMIC WEIGHTS OF THE ELEMENTS 1979
N.E. Holden, Chairman (USA), R.L. Martin, Secretary (Australia), R.C. Barber
(Canada), I.L. Barnes (USA), P.J. De Bivre (Belgium), R. Hagemann (France), W.H.
Johnson (USA), T.J. Murphy (USA), Associate Members: A.E. Cameron (USA), J.R. de
Laeter (Australia), S. Fujiwara (Japan), R. Gonfiantini (Italy), N.N. Greenwood
(UK), 'z. Horibe (Japan), H.S. Peiser (USA). National Representative: V.1.
Goldanskii (USSR).
Inorganic Chemistry Division, Commission on Atomic Weights and Isotopic Abundances
Abstract — The biennial review of atomic weight determinations and other cognate
data has resulted in the following changes in recommended values (1977 values in
parentheses): Neon 20.179 (20.179*), Argon 39.948 (39.948*), Potassium 39.0983
(39.0983*), Titanium 47.88* (47.90*), Nickel 58.69 (58.70), Palladium 106.42
(106.4), Xenon 131.29* (131.30), Samarium 150.36* (150.4), Tantalum 180.9479
(180.9479*), Platinum 195.08* (195.09*), Thallium 204.383 (204.37*), Uranium
238.0289 (238.029). These values are considered to be reliable to ± in the last
digit or ±3 when followed by an asterisk (*) and are incorporated in the full Table
of Atomic Weights of the Elements 1979. The Report outlines various problems which
arise from the present imprecise definition of 'atomic weight (mean relative atomic
mass)" and contains a new definition to overcome the difficulties. The importance
of having informative labels on commercially available chemicals is emphasized, par—
ticularly in order to warn or reassure users of the presence or absence of mate—
rials containing elements with unusual atomic weights due to the enrichment or de—
pletion of isotopes. The Report includes a complete review of the natural isotopic
composition of the elements and also tabulates the Relative Atomic Masses for Se—
lected Radioisotopes. The Report contains a review of stable isotope abundances of
elements from non—terrestrial sources.
INTRODUCTION
The Commission on Atomic Weights met under the chairmanship of Professor E. Roth, on 3—6 Sep—
tember 1979, during the XXXth IUPAC General Assembly in Davos, Switzerland. Work done by
the Commission members during the preceding two years in assessing atomic weights and other
cognate data was reviewed and, as a result, the recommended values for the atomic weights of
twelve elements were changed and footnotes added for two elements. The new values were immediately disseminated through an IUPAC News Release. The justifications for these changes
are set out in the next section. This is followed by the definitive Table of Standard
Atomic Weights of the Elements 1979 of the International Union of Pure and Applied Chemistry.
General problems of terminology are discussed in the next section, and the Commission has a
new definition of "atomic weight (mean relative atomic mass)." It is hoped that this will
remove various operational difficulties which at present face the Commission in preparing
its recommendations for the atomic weights of the elements, and place the whole concept of
an atomic weight on a sounder basis.
An increasing number of commercially available materials contain elements whose isotopic composition has been altered, either intentionally or inadvertently, from that of the element
in nature This problem afflicts some elements more than others and the Commission has been
both manufacturers and suppliers to the need for appropriate
phrases on labels. Suggestions are made for such explanatory statements which, in many
cases, may well add to the usefulness of the products described.
A working group had been constituted as a Subcommittee for the Assessment of Isotopic Composition, at the request of the Inorganic Division (the parent body of the Commission). This
will, in due course, enable the Commission to publish a completely self—consistent set of
isotopic compositions and atomic weights of the elements incorporating not only mass—
spectrometric data but also results obtained from all other relevant methods. The present
Report tabulates the range of published mass—spectrometrically determined isotopic abundances for each of the naturally occurring elements, together with the result of what is con—
silered to be the best available mass—spectrometric measurement for a single natural source
of each element, and a representative value for the isotopic composition for average
elemental. properties. This best mass—spectrometric measurement is not necessarily a good
one in terms of 1979 knowledge nor does it necessarily provide the best atomic weight value
in terms of all techniques. In future years the definitive self—consistent tabulation of
isotopic compositions will also include a precise relative atomic mass of each nuclide and
this will obviate the need for their separate tabulation. As an interim measure, however,
the present Report continues the practice of tabulating the relative atomic masses of selected nuclides, but restricts these to certain nuclides of radioactive elements, including
active in seeking to alert
2351
COMMISSION ON ATOMIC WEIGHTS AND ISOTOPIC ABUNDANCIES
2352
those such as technetium, promethium, and the elements of highest atomic number, for which
the Table of Atomic Weights lists only an atomic mass number in parentheses. The Report tab—
ulates examples of variations in isotopic composition for selected elements in non—
terrestrial samples along with an introductory discussion of this topic of increasing
interest.
CHANGES IN ATOMIC WEIGHT VALUES
Ne on
The value of r)20183 for the atomic weight of neon was adopted by the Atomic Weights
Ccxnmission in its 1961 Report (Ref. 1) based on gas density measurements by Baxter and
Starkweather (Ref. 2) and Baxter (Ref. 3). In its 1967 Report, the atomicweight was
changed to r(Ne)2O.179±O3 on the basis of two calibrated isotopic composition determina—
tions which appeared almost simultaneously. Eberhardt (Ref. 4) prepared one standard by
mixing known amounts of atmospheric neon with 20Ne enriched to 99.70%. They also recovered
neon from air without distillation. Walton and Cameron (Ref. 5) prepared five usable
standards from separated neon isotopes of high purity. Six samples of neon from commercial
suppliers, separated from air at different times were run alternately with a standard. No
significant differences were observed among the samples within the limits of error of the
measurements. Based on the agreement between the two calibrated measurements and the ab—
sence of observed variations, the Ccxnmission recormnends Ar(Ne)20.179±O.OO1 as the most
reliable value.
Argon
The value of r139942 (converted to the '2C scale) for the atomic weight of argon was
adopted by the Atomic Weights Commission based on gas density measurements by Baxter and
Starkweather (Ref. 6) believed by the workers to be good to ±0.001. In its 1961 Report
(Ref. 1) the atomic weight was changed to r()39.948±O3 on the basis of a calibrated
measurement of isotopic composition from carefully prepared mixture of 36Ar and 40Ar of high
isotopic purity by Nier (Ref. 7). The Commission has reexamined the calibrated measurements
by Nier and now recommends Ar(Ar)39.948±O.OO1 with the lowered uncertainty as the most reli—
able value for argon.
Potassium
The value of Ar(K)=39.102 for the atomic weight of potassium was adopted by the Atomic
Weights Commission in its 1961 Report (Ref. 1) based on the mass—spectrometric measurements
of Nier (Ref. 8). In the 1969 Report (Ref. 9), the Commission noted that this value was
known "less reliably" and introduced the uncertainty of ±0.003. In the 1971 Report (Ref.
10); the Commission recommended a change to 39.098±.003 based on a new analysis by Marinenko
(Ref. 11) of older data by Bates and Wickers (Ref. 12). In the 1975 Report (Ref. 13), the
Commission recommended r(K)39.0983±00003 based on the absolute measurement and survey of
possible variations by Garner et al. (Ref. 14). The Commission has now completed an evaluation of possible variations of the isotopic abundances and the effects of small errors in
the abundance measurements and recommends the present value with a reduced uncertainty of
r(I0 =39 . 0983±0. 0001.
Titanium
The value of Ar(Ti)47.90 for the atomic weight of titanium was adopted by the Atomic
Weights Commission in 1927 based on the chemical determinations of Baxter and Butler (Ref.
15) and (Ref. 16). In its 1969 Report (Ref. 9), the Commission examined the uncertainty on
the above value and recommended Ar(Ti)47.90±0.03 based on Baxter with some consideration of
the isotopic abundance measurements, by Nier (Ref. 17), Hibbs (Ref. 18), Mattraw (Ref. 19),
Hogg (Ref. 20), Drawin (Ref. 21), and Belsheim (Ref. 22).
The Commission has reexamined both the chemical and the mass—spectrometric determinations
and recommends Ar(Ti)47.88±0.03 for the atomic weight of titanium, which includes the above
references but is weighted toward the calibrated measurement of Belsheim. The uncertainty
covers both the mass—spectrometric determinations and the chemical measurement.
Nickel
The value of r(1)58.69 for the atomic weight of nickel was adopted by the Atomic Weights
Commission in 1925 based on the measurement of the ratios NiO/Ni by Baxter and Parsons (Ref.
23) and of NiCl2/2 AgCl by Baxter and Hilton (Ref. 24). These were confirmed by the work of
Baxter and Ishimaru (Ref. 25) on the ratios NiBr2/2Ag and NiBr2/2AgBr. In 1955, the atomic
weight was changed to Ar(Ni)58.71 based on the isotopic abundance measurement of White and
Cameron (Ref. 26). In their 1969 Report (Ref. 9), the Commission introduced an uncertainty
of 0.03 to encompass both the physical and chemical values. Concerned that the 64Ni abundance may have been overestimated by White and Cameron, the Commission recommended an atomic
weight value of 58.70±0.01 in its 1973 Report (Ref. 27). The Commission has reviewed both
the chemical and mass—spectrometric determinations including the newer, more precise measurement by Barnes et al. (Ref. 28) and now recommends an atomic weight for nickel of
r58.69001
Atomic weights of the elements 1979
2353
Palladium
The value of r()6.4 for the atbmic weight of palladium was adopted by the Atomic
Weights Ccxnmission in its 1961 Report (Ref. 1) based on the isotopic abundance measurement
of Sites et al. (Ref. 29). In its 1969 Report (Ref. 9), the Commission considered the uncer—
tainty on this value and recommended r()1O6.4±0.1. A new calibrated measurement of the
isotopic abundance values of palladium has been made by Shima et al. (Ref. 30). Using these
new abundance values and the evidence of lack of significant natural variations, the Cominis—
sion now recommends r(Pd)lO642±M as the most reliable value.
Xenon
The value of r()l3L3 for the atomic weight of xenon was adopted by the Atomic Weights
Coimnission in 1932 (Ref. 31) based on the measurements by Whytlaw—Gray et al., of the ratio
of the pressures at which the densities of xenon and oxygen were equal (Ref. 32). This
value was supported by the xenon isotopic composition measurement by Aston (Ref. 33) with
the mass spectrograph. In 1955, the Commission reconinended Ar(Xe)l30.30 (on the 016
scale) based on the isotopic composition measurement by Nier (Ref. 34) and the atomic mass
measurement of Halsted (Ref. 35). In their 1961 Report, the Commission continued the same
value which was based on Nier's abundance values and atomic masses from Mattauch (Ref. 36)
and known to be slightly in error. This report states, "With the same abundances and the
masses from EKMW (1960), the calculated atomic weight is 131.29. The Commission recommended
131.30 for the present table, based on an earlier calculation which was slightly in error."
The Commission now corrects that error and recommends Ar(Xe)131.29±O.03 for the atomic
weight of xenon.
Samarium
In 1955, the Atomic Weights Commission reconinended Ar(Sm)150.35 for the atomic weight of
samarium based upon the isotopic abundance measurement by Inghram et al. (Ref. 37) and
atomic masses of Hogg and Duckworth (Ref. 38). This value was revised to
in the 1969 Report (Ref. 9) based on an evaluation of the uncertainty in the previously rec—
oninended value. After a critical review of the high precision chemical measurement of
Hnigschmid and Hirschbold—Wittner (Ref. 39) and the mass—spectrometric measurements by
Inghram and Lugmair et al. (Ref. 40), the Commission now recotrnnends Ar(Sm)150.36±O.03.
Tantalum
A very precise atomic weight value can be expected for this element because it has two
isotopes, one of which is overwhelmingly predominant. In their 1969 Report (Ref. 9), the
Atomic Weights Commission recomended Ar(Ta)180.9479±O.0003 for the atomic weight of tantalum based on the isotopic abundance measurements of White et al. (Ref. 41, 42) and of Palmer
(Ref. 43). The Commission has reviewed the published measurements and uncertainties and now
recommends Ar(Ta)l80.9479±O.000l as the most reliable value.
Platinum
The value of Pt)l95.O9 for the atomic weight of platinum was adopted by the Commission
in 1955 based upon isotopic abundance measurements by Inghram et al. (Ref. 44) and Leland
(Ref. 45), and masses measured by Duckworth et al. (Ref. 46). This value had a
calculational error. With the correct conversion factor between chemical and physical
scales, the atomic weight should be (Pt)195.O8. The Commission has reviewed the
published data on recomends Ar(Pt)195.08±O.03 as the most reliable values based on White's
isotopic composition (Ref. 42Y and Wapstra's atomic masses (Ref. 47).
Thallium
The value of (Tl)2O4.39 for the atomic weight of thallium was adopted by the Commission
in 1925 based on the measurement of the combining weights of T1C1/Ag and T1C1/AgCl by
Hnigschmid et al. (Ref. 48). Later work by Hnigschmid and Striebel (Ref. 49) gave an
identical value. On the '2C scale, this value was recalculated to 204.37. In the 1961 Report (Ref. 1), the Commission recommended the value 204.37±0.03, although the mass—
spectrometric determinations of White and Cameron (Ref. 26) and Hibbs (Ref. 50) gave a value
of 204.38. Dunstan et al. (Ref. 51) reports a calibrated measurement and survey of thallium
materials and minerals which indicate no variation in nature. The Commission now recommends
r(Tl)204.383±O001 as the most reliable value.
Uranium
A relatively precise atomic weight value can be expected for uranium because one of its
three isotopes is predominant. In 1937, the Commission reconinended r(1)238.07 based on
the measurements of the ratio UC14/4Ag by Hnigschmid and Wittner (Ref. 52). On the '2C
scale, this value recalculates to 238.05. In the 1961 Report (Ref. 1), the Commission
recommended r 1)238.03 based on the isotopic abundance measurements of White (Ref. 42),
and Boardman and Meservey (Ref. 53), the variations reported by Smith (Ref. 54) and Senftle
et al. (Ref. 55) and the atomic masses of Mattauch (Ref. 36). After a review of
uncertainties, the Commission reconinended the value 238.029±0.001 in the 1969 Report (Ref.
9). The Commission has now reviewed published data on isotopic compositions, and the 235U
variation in nature by Cowan and Adler (Ref. 56) and the 234U variation in nature by Smith
2354
COMMISSION ON ATOMIC WEIGHTS AND ISOTOPIC ABUNDANCIES
235
and Jackson (Ref. 57). Based on the range of
U (0.7198—0.7202 atom percent) and the
range of 234U (0.00509—0.00548 atom percent), the Commission now recommends
(u)=238.O289±O.ooo1 for natural uranium.
CHANGES IN FOOTNOTES
Hydrogen
As mentioned in the 1977 Report (Ref. 58), the atomic weight of hydrogen has a recommended
value of 1.0079±0.0001, while electrolytic hydrogen (Ref. 59), and Russian water source
(Ref. 60) have deuterium contents which lead to atomic weight values which are outside the
range, 0.0001, of an atomic weight value of either 1.0080, or 1.0079, respectively. The Corn—
mission retains the previously recommended atomic weight but now adds the footnotes x and y
to account for the above mentioned cases. Hydrogen will be reconsidered in the overall re—
view in 1981 when the Commission revises the policy on quoted uncertainties on atomic
weights.
Oxygen
Oxygen is another element for which the uncertainty presents a problem. The reccinmended
atomic weight value Ar(O)15.9994±O.0003 is based on the isotopic composition in the atmo—
sphere as measured by Nier (Ref. 7) and reanalyzed by Craig (Ref. 61). Lorius (Ref. 62)
measured the 180/160 value in antarctic ice, which corresponds to an atomic weight of
15.9990 outside the quoted range. The Ccinrnission adds the footnote x to account for this
source of oxygen.
THE TABLE OF STANDARD ATOMIC WEIGHTS 1979
The changes listed in the previous Section are incorporated in the 1979 Table of Standard
Atomic Weights (see next section). As has been customary, the Table is presented, firstly,
in alphabetic order by English names of the elements (Table 1) and, secondly, in order of
atomic numbers (Table 2). This year, the Coimnission's Subcommittee for the Assessment of
Isotopic Composition (SAIC) has carefully reviewed all significant experimental and interpre—
ative evidence bearing on atomic weights for all the elements. The results of this study
are the above changes in atomic weight values and footnotes.
The need for new and better atomic weight determinations is felt as strongly as ever. The
margin in precision between the best atomic weight determinations and the results of
routinely available analytical techniques is shrinking and is nonexistent for elements such
as Zn and Ge. The Commission notes work underway on the atomic weight of silver which directly affects the determination of the Faraday constant.
TERMINOLOGY
Previous discussions by the Commission on Atomic Weights (see especially the 1975 Report
(Ref. 13)) have revealed difficulties arising from the current definition of "atomic
weight." These stem from the fact that, for some elements, the atomic weight value stated
to the precision available with present experimental techniques can differ for different
samples, because these elements occur with different isotopic composition (in nature or by
artificial alteration). In some fields of modern chemistry and technology an operational
problem therefore exists which can no longer be disregarded. Such different "atomic weight"
values are more precise than indicated by the uncertainties associated with the present defiof atomic weight. At the 1975 IUPAC General Assembly in Madrid, and the 1977 assembly in Warsaw, the Commission received the comments and advice from an Open Meeting
conducted in cooperation with the IUPAC Inorganic Division, the Interdivisional Committee on
Education and other IUPAC commissions concerned with terminology. After those open
meetings, the Atomic Weights Commission accepted the responsibility to propose a new definition of an atomic weight of an element at the 1979 Davos General Assembly. At a joint meeting in Davos of IUPAC Commissions on Inorganic Nomenclature, Atomic Weights, Organic Nomenclature, Analytical Nomenclature, Physico—Chemical Symbols, Terminology and Units, Committee
on Teaching of Chemistry and the Interdivisional Committee on Nomenclature and Symbols, a
new definition resulted.
The definition of an atomic weight (mean relative atomic mass) of an element from a
specified source is "The ratio of the average mass per atom of the element to 1/12 of the
mass of an atom of '2C."
Remarks on the definition:
(1) Atomic weights can be defined for any sample.
(2) Atomic weights are evaluated for atoms in their electronic and nuclear ground states.
(3) The "average mass per atom" in a specified source is the total mass of the element divided by the total number of atoms of that element.
(4) Dated Tables of Standard Atomic Weights published by the Commission refer to our best
knowledge of the elements in natural terrestrial sources.
The new definition by itself does not solve the principal problem of the Commission namely
how to present the most accurate available values for those who need to use them. The con—
nition
2355
Atomic weights of the elements 1979
cept of accuracy implies the existence of a true value and the definition purposely denies
or at any rate fails to recognize the existence of one true value for every element.
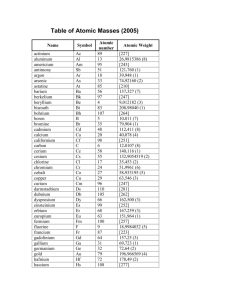
TABLE 1. Standard Atomic Weights 1979
(Scaled to the relative atomic mass, Ar(12C)=l2)
The atomic weights of many elements are not invariant but depend on the origin and treatment
of the material. The footnotes to this Table elaborate the types of variation to be expected for individual elements. The values of .r(E) given here apply to elements as they
exist naturally on earth and to certain artificial elements. When used with due regard to
the footnotes they are considered reliable to ±1 in the last digit or ±3 when followed by an
asterisk*. Values in parentheses are used for radioactive elements whose atomic weights cannot be quoted precisely without knowledge of the origin of the elements; the value given is
the atomic mass number of the isotope of that element of longest known half life.
Alphabetical order in English
Name
Symbol
Atomic
number
Atomic
weight
227.0278
26.98154
Actinium
Aluminium
Americium
Ac
Al
89
Am
Sb
95
51
(243)
Antimony (Stibium)
Argon
Ar
As
At
Ba
Bk
Be
Bi
18
39.948
74.9216
(210)
137.33
(247)
9.01218
208.9804
10.81
79.904
112.41
132.9054
Arsenic
Astatine
Barium
Berkelium
Beryllium
Bismuth
Boron
Bromine
Cadmium
Caesium
Calcium
Californium
Carbon
Cerium
Chlorine
Chromium
Cobalt
Copper
Curium
Dysprosium
Einsteinium
Erbium
Europium
Fermium
Fluorine
Francium
Gadolinium
Gallium
Germanium
Gold
Hafnium
Helium
Holmium
Hydrogen
Indium
Iodine
Iridium
Iron
Krypton
Lanthanum
Lawrencium
Lead
Lithium
Lutetium
Magnesium
Manganese
Mendelevium
B
13
33
85
56
97
4
83
Br
5
35
Cd
48
Cs
55
20
98
6
58
17
Ca
Cf
C
Ce
Cl
Cr
Co
Cu
Cm
Dy
Es
Er
Eu
Fm
F
24
27
29
96
66
99
68
63
100
9
Fr
Gd
Ga
Ge
Au
87
64
Hf
He
72
2
Ho
67
H
In
I
Ir
Fe
Kr
31
32
79
1
49
53
77
26
36
57
La
Lr
103
Pb
82
Li
3
Lu
Mg
Mn
Md
71
12
25
101
Footnotes
z
121.75*
40.08
(251)
12.011
140.12
35.453
51.996
58.9332
63.546*
(247)
167.50*
w x
x
w y
x
x
w
x
w
(252)
167.26*
151.96
(257)
18.998403
(223)
x
157.25*
69.72
72.59*
196.9665
178.49*
4.00260
164.9304
1.0079
114.82
x
x
w x y
x
126.9045
192.22*
55.847*
83.80
138.9055*
(260)
207.2
6.941*
174.967*
24.305
54.9380
(258)
x y
x
w x
w x y
x
COMMISSION ON ATOMIC WEIGHTS AND ISOTOPIC ABUNDANICES
2356
TABLE 1.
Name
Mercury
Molybdenum
Neodymium
Neon
Neptunium
Nickel
Niobium
Nitrogen
Nobelium
Osmium
Oxygen
Palladium
Phosphorus
Platinum
Plutonium
Polonium
Potassium (Kalium)
Praseodymium
Promethium
Protactinium
Radium
Radon
Rhenium
Rhodium
Rubidium
Ruthenium
Samarium
Scandium
Selenium
Silicon
Silver
Sodium (Natrium)
Strontium
Sulfur
Standard Atomic Weights 1979 (cont'd)
Symbol
Hg
Mo
Nd
Ne
Np
Ni
Nb
N
No
Os
0
Pd
P
Pt
Pu
Po
80
42
60
10
93
28
41
7
102
76
8
46
15
K
78
94
84
19
Pr
Pm
Pa
59
61
91
Ra
Rn
Re
88
Rh
Rb
Ru
Sm
45
Sc
Se
Si
21
34
14
Ag
47
Na
11
38
16
73
Sr
S
Tantalum
Technetium
Tellurium
Terbium
Thallium
Thorium
Thulium
Tin
Titanium
Tungsten (Wolfram)
Ta
Tc
(Unnilhexium)
(Unnilpentium)
(Unnilquadium)
Uranium
(Unh)
(Unp)
(Unq)
Vanadium
Xenon
Ytterbium
Yttrium
Zinc
Zirconium
Atomic
number
Te
Yb
Tl
Th
Tm
Sn
Ti
W
U
V
86
75
weight
95.94
144.24*
20.179
237.0482
58.69
92.9064
14.0067
(259)
190.2
15.9994*
106.42
30.97376
195.08*
(244)
(209)
39.0983
140.9077
(145)
231.0359
226.0254
(222)
186.207
102.9055
37
85.4678*
101.07*
150.36*
44.9559
78.96*
28.0855*
107.868
22.98977
43
52
65
81
90
69
50
22
74
106
105
104
92
Yb
Y
39
Zn
Zr
30
40
Footnotes
200. 59*
44
62
23
54
70
Xe
Atomic
x
y
z
x
w x
x
x
x
x
x
x
8.62
32.06
180.9479
z
z
x
w
(98)
127.60*
158.9254
204.383
232.0381
168.9342
118.69*
47.88*
183.85*
(263)
(262)
(261)
238.0289
50.9415
131.29*
173.04*
88.9059
65.38
91.22
x
x
z
x y
x y
x
w Element for which known variations in isotopic composition in normal terrestrial material
prevent a more precise atomic weight being given; (E) values should be applicable to
any "normal" material.
x
Element for which geological specimens are known in which the element has an anomalous
isotopic composition, such that the difference between the atomic
weight of the element
in such specimens and that given in the Table may exceed considerably the implied
uncertainty.
y Element for which substantial variations in
from the value given can occur in
coninercially available material because of inadvertent or undisclosed change of isotopic
composition.
z Element for which the value of Ar is that of the radioisotope of longest half—life.
2357
Atomic weights of the elements 1979
TABLE 2.
Standard Atomic Weights 1979
(Scaled to the relative atomic mass Ar(12C) = 12)
The atomic weights of many elements are not invariant but depend on the origin and treatment
of the material. The footnotes to this Table elaborate the types of variation to be ex(E) given here apply to elements as they
pected for individual elements. The values of
exist naturally on earth and to certain artificial elements. When used with due regard to
the footnotes they are considered reliable to ±1 in the last digit or ±3 when followed by an
asterisk.* Values in parentheses are used for radioactive elements whose atomic weights cannot be quoted precisely without knowledge of the origin of the elements; the value given is
the atomic mass number of the isotope of that element of longest known half life.
Order of Atomic Number
Atomic
Number
1
2
3
4
Name
Symbol
Hydrogen
Helium
Lithium
Beryllium
H
He
Li
Be
5
6
7
8
9
10
Boron
Carbon
B
C
Nitrogen
Oxygen
Fluorine
Neon
N
0
11
Sodium (Natrium)
12
13
14
15
16
17
18
19
Magnesium
Aluminium
20
21
22
23
24
25
Silicon
Phosphorus
Sulfur
Chlorine
Argon
Potassium (Kalium)
Calcium
Scandium
Titanium
Vanadium
Chromium
Manganese
26
Iron
27
Cobalt
Nickel
Copper
28
29
30
31
32
33
34
35
36
37
38
39
40
41
42
43
44
45
46
47
48
49
50
51
52
53
54
55
Zinc
Gallium
Germanium
Arsenic
Selenium
Bromine
Krypton
Rubidium
Strontium
Yttrium
Zirconium
Niobium
Molybdenum
Technetium
Ruthenium
Rhodium
Palladium
F
Ne
Na
Mg
Al
Si
P
S
Cl
Ar
K
Ca
Sc
Ti
V
Cr
Mn
Fe
Co
Ni
Cu
Zn
Ga
Ge
As
Se
Br
Kr
Rb
Sr
Y
Zr
Nb
Mo
Atomic
Weight
1.0079
4.00260
6.941*
9.01218
10.81
12.011
14.0067
15.9994*
18.998403
20.179
22.98977
24.305
26.98154
28.0855*
30.97376
32.06
35.453
39.948
39.0983
40.08
44.9559
47.88*
50.9415
51.996
54.9380
55.847*
58.9332
72.59*
74.9216
78.96*
79.904
83.80
85.4678*
87.62
88.9059
91.22
92.9064
95.94
(98)
101.07*
102.9055
106.42
107.868
112.41
114.82
118.69*
121.75*
127.60*
Pd
Cadmium
Indium
Tin
Antimony (Stibium)
Tellurium
Cd
In
Sn
Sb
Te
Icine
I
Xenon
Caesium
Xe
Cs
x
w x y
w y
w
w x
y
x
w
w x
x
w
69.72
Ru
Rh
Ag
w x y
58.69
63.546*
65.38
Tc
Silver
Footnotes
x y
x
x
x
x
x
x
x
x
x
126.9045
131.29*
132.9054
x y
2358
COMMISSION ON ATOMIC WEIGHTS AND ISOTOPIC ABUNDANCIES
TABLE 2. Standard Atomic Weights 1979 (cont'd)
Atomic
Number
56
57
58
59
60
61
62
63
64
65
66
67
68
69
70
71
72
73
74
75
76
77
78
79
80
81
82
83
84
85
86
87
88
89
90
91
92
93
94
95
96
97
98
99
100
101
102
103
104
105
106
Name
Barium
Lanthanum
Cerium.
Praseodymium
Neodymium
Promethium
Samarium
Europium
Gadolinium
Terbium
Dysprosium
Holmium
Erbium
Thulium
Ytterbium
Lutetium
Hafnium
Tantalum
Wolfram (Tungsten)
Rhenium
Symbol
Ba
La
Ce
Pr
Nd
Pm
Sm
Eu
Am
(243)
Cm
Bk
Cf
Es
Fm
(247)
(247)
(251)
Md
No
(258)
(259)
(260)
(261)
(262)
(263)
Th
Dy
Ho
Er
Tm
Yb
Lu
Hf
Ta
W
Iridium
Ir
Platinum
Gold
Mercury
Thallium
Lead
Pt
Bismuth
Bi
Au
Hg
Tl
Pb
Po
At
Rn
Fr
Ra
Ac
Th
Pa
U
Lr
(Unq)
(Unp)
(Unh)
Footnotes
x
x
x
x
(145)
Np
Pu
Gd
Re
Os
(Unnilquadium)
(Unnilpentium)
(Unnilhexium)
137.33
138.9055*
140.12
140.9077
144.24*
150.36*
151.96
157.25*
158.9254
162.50*
164.9304
167.26*
168.9342
173.04*
174.967*
178.49*
180.9479
183.85*
186.207
190.2
192.22*
195.08*
196.9665
200.59*
204.383
207.2
208.9804
(209)
(210)
(222)
(223)
226.0254
227.0278
232.0381
231.0359
238.0289
237.0482
(244)
Osmium
Polonium
Astatine
Radon
Francium
Radium
Actinium
Thorium
Protactinium
Uranium
Neptunium
Plutonium
Americium
Curium
Berkelium
Californium
Einsteinium
Fermium
Mendelevium
Nobelium
Lawrencium
Atomic
Weight
x
x
x
x
w x
x
x
z
z
z
z
x y
z
(252)
(257)
w Element for which known variations in isotopic composition in normal terrestrial material
prevent a more precise atomic weight being given; Ar(E) values should be applicable to
any "normal" material.
x Element for which geological specimens are known in which the element has an anomalous
isotopic composition, such that the difference between the atomic weight of the element
in such specimens and that given in the Table may exceed considerably the implied
uncertainty.
y Element for which substantial variations in Ar from the value given can occur in
commercially available material because of inadvertent or undisclosed change of isotopic
composition.
z Element for which the value of Ar is that of the radioisotope of longest half—life.
Atomic weights of the elements 1979
2359
LABELLING OF WELL CHARACTERIZED MATERIALS
As pointed out in the 1975 and 1977 Reports (Ref. 13,58) the Commission is concerned that
the useful practice of quoting atomic or molecular weights on bottles could be misleading
for compounds prepared from residues of an undisclosed isotope separation process. One of
the following statements continues to be recommended when additional labelling is judged ad—
visable to avoid possible misconceptions or errors by the user, or to reassure the user of
the "normality" of the material.
(1) Atomic weights conform with values published in the IUPAC Table of Standard Atomic
Weights. (It might be considered desirable, though not essential, to include the date of
the IUPAC Table referred to.)
(2) The actual atomic weights of element(s). . . .in this particular sample is (are). . . .(In
this statement "atomic weight(s)" could be replaced by "isotopic composition(s).")
(3) Element X is enriched (depleted) in isotope X.
In some materials statement (1) can be applied to some elements and statement (2) can be
made for one or more other elanents in the same sample. Probable error limits would often
be helpful in statement (2), and also in statement (3) when it is combined with quantitative
data expressed as isotopic composition. Some manufacturers have already started quoting iso—
topic composition on their labels. The Reagents Committee of the American Chemical Society
has already added a warning to reagent grade uranium, boron and lithium chemicals.
The Commission has requested the widest possible dissemination of these proposals and
welcomes comments especially before its next meeting in 1981. Such coimnents and related
questions should be directed to the Commission's Secretary, Prof. R.L. Martin, Vice Chancel—
br, Monash University, Clayton, Victoria, 3168, Australia.
THE ISOTOPIC COMPOSITION OF THE ELEMENTS
At the request of the IUPAC Inorganic Division, a Subcommittee for the Assessment of Isotop—
ic Composition (SAIC) was formed within the Commission on Atomic Weights and Isotopic Abun—
dances (Ref. 13). SAIC is concerned with all measurements for deriving isotopic
compositions. SAIC has produced another interim version of the "Table of Isotopic Composi—
tions of the Elements as Determined by Mass Spectrometry," and it is reproduced here (Table
3). The interim values when converted to atomic weights are not all fully consistent with
the 1979 Table of Standard Atomic Weights. Discrepancies are most noticeable in the cases
of zinc, germanium, and selenium where the interim values lie outside the limit of uncer—
tainty on the recommended atomic weight. For germanium, this corresponds to a difference of
0.06%.
For the 1981 meeting of the Commission, SAIC has been asked to include uncertainties from ±1
to ±9 on all isotopic compositions and atomic weights.
Present members of SAIC are P. De Bivre (Chairman), I.L. Barnes, A.E. Cameron, R.
Hagemann, N.E. Holden and H. Thode. Additional assistance has been provided by E. Roth,
H.S. Peiser and T.J. Murphy.
The Commission thanks SAIC for its efforts in preparing Table 3.
NON-TERRESTRIAL DATA
The values of stable isotope abundances of elements from non—terrestrial sources form a rich
and rapidly expanding body of information. Many significant variations from normal terres-
trial isotopic abundance values have already been reported and more will undoubtedly be
found in the future. The intention of the Commission on Atomic Weights and Isotopic Abundances to tabulate non—terrestrial isotopic abundances was signaled in the 1977 Report (Ref.
58). For the present Report, it seems appropriate to illustrate some of the most significant variations with the intention of considering later the completion of a more comprehensive listing.
Information about non—terrestrial isotopic abundances comes from several sources. The study
of meteoritic materials provided the earliest samples of non—terrestrial material for direct
analysis. More recently, the analysis of lunai samples has produced a large number of new
results. Space probes carrying mass spectrometers or other abundance measuring equipment
have been employed to analyze the atmospheres and surface materials of other planets and
satellites. Finally, earth—based optical observations of various astronomical objects have
led to the determination of some isotopic abundances for these objects.
There are many processes which can alter isotopic abundances. Firstly, there is mass
fractionation, where the rate of a process is dependent on the mass of the atoms or molecules involved in the process. This mass fractionation will result from either unidirectional or equilibrium processes. This category included chemical reactions as well as
processes dependent on thermal gradients, pressure gradients, interaction with electric, magnetic fields, diffusion fields or gravitational fields. A systematic variation in isotopic
abundance for a series of isotopes in a multiisotopic element is usually considered to be
sufficient proof that mass fractionation has occurred.
Secondly, isotopic abundances can be modified in various specific nuclear reactions. For example, there is the possibility that variations in the nucleosynthesis processes involved
in element building may result in differing isotopic abundances for elements in matter from
P.A.A.C.
52/10—H
2360
CONNISSION ON ATOMIC WEIGHTS AND ISOTOPIC ABUNDANCIES
TABLE OF ISOTOPIC COMPOSITIONS AND ATOMIC WEIGHTS AS DETERMINED
BY MASS SPECTROMETRY
Introduction
The Subcommission for the Assessment of Isotopic Composition (SAIC) has
examined all of the literature available to it through August, 1979. The
Subcommission has evaluated this data to produce a table of recommended
isotopic abundances for the elements and the atomic weights calculated from
these abundances. The table is intended to include values for terrestrial
samples only and does not include values published for meteoritic or other
extra terrestrial materials. A description of the contents of each of the
columns contained within this table is given below:
Column Headings
Column 1:
The atomic numbers of the elements are given in ascending order.
Column 2:
The names of the elements are listed using the abbreviations
recommended by IUPAC.
Column 3:
The mass number for each elemental isotope is listed.
Column 4:
Given are the highest and lowest abundances published for each
isotope from measurements which have been evaluated and accepted
by the Subcommission. The range given includes natural variations but does not include values for certain, exceptional, or
unusual samples (these are noted with a "G" in column 5). No
data are given in this column when the absence of a range has
been, in the opinion of SAIC, reliably established.
Column 5:
The letters appended in this column have the following
significance:
"R" is appended when the range given corresponds to that of
established natural variations.
"D" is appended when the range corresponds to differences
between published values not supported by established natural
variations.
"G" is appended when the element is known to have an anomalous
composition in certain, natural terrestrial specimens.
"X" is appended when data from only one measurement are
availgble.
"I" is appended when, as a result of reliable surveys, the
isotopic composition is not believed to vary in terrestrial
samples within the limits established. Though "I" is appended
there may be rare or unusual samples where the values may
differ and a "G" is also appended.
Column 6:
In this column are given the data from the best measurement of a
sample from a single terrestrial source. The values are
reproduced from the original literature. The values given in
parenthesis are the errors on the last corresponding digits and
are given as in the original publication. Where no errors are
given none were available in the literature. The errors are,
of course, not given in any uniform manner in the literature
and SAIC indicates this as follows: l,2,3o indicates 1, 2, or
3 sigma or standard deviations, P indicates probable error (as
defined by the author) and SE indicates standard error. "C"
is appended when the measurement has been calibrated and is
thus believed to be "absolute" within the errors stated in the
original publication. The user is cautioned that since the
data are reproduced from the literature the sum of the isotopic
abundances may not be equal to 100 percent. The user is also
cautioned that, when a range of compositions has been
established, the samples used for the best measurement may come
from any part of the range. Attention is drawn to the fact
that a "Best Measurement" is not necessarily a good one in
SAIC's opinion.
Atomic weights of the elements 1979
2361
Column 7:
The reference to the literature containing the best measurement
is given. The complete citation is given in Appendix A.
Column 8:
Reference materials or samples which are known to be available
and which relate to the best measurement are listed. Where one
or more materials are available which represent the best
measurement, these are marked with an asterisk. Additional
information is contained in Appendix B.
Column 9:
In this column are listed the values for the isotopic composition
of the elements which, in the opinion of SAIC, will include the
chemicals and/or materials most commonly encountered in the
laboratory. They may not, therefore, correspond to the most
abundant natural material. For example, in the case of hydrogen,
the deuterium content quoted corresponds to that in fresh water
in temperate climates rather than to ocean water. The uncertainties listed in parenthesis cover the range of probable
variations of the materials as well as experimental errors.
Uncertainties quoted are from one to nine in the last digit
except for a few cases where rounded values would be outside of
the observed range. In those cases uncertainties greater than
nine have been used.
Warning
1)
2)
Representative isotopic compositions should not be used for
other than average properties.
The reader is reminded that for more precise work, as for
example to work out individual properties, samples with
more precisely known isotopic abundances (such as those
listed in column 8) should be obtained or suitable
measurements should be made.
Column 10: Listed are the atomic weights and uncertainties calculated from
the data in the preceding column. For these calculations
nuclidic masses were used as given by: A. H. Wapstra and
K. Bos, "The 1977 Atomic Mass Evaluation", Atomic Data and
Nuclear Data Tables, 19, 177 (1977). The values listed for
mononuclidic elements are taken from the same source with the
given uncertainties multiplied by a factor of six.
3
4
6
14
15
16
17
18
19
20
21
22
23
He
Li
Be
B
C
N
0
F
Ne
Na
Mg
2
3
4
5
6
7
8
9
10
11
12
24
25
26
90.514
1.71
9.96
99.7771
0.0407
0.2084
99.639
0.375
98.99
1.14
12
13
20.316
80.902
7.65
92.70
0.0041
100
99.9918
0.0230
10
11
9
7
2
1
H
Element
Mass
Number
1
Atomic
Number
0.361
99.625
98.86
1.01
19.098
79.684
-
-
9.20
88.47
0.266
0.035
0.1879
- 99.7539
-
-
-
-
-
-
7.30
-
92.35
99.9959
6x108
99.9770
0.0082
-
-
-
-
Evaluated
Range of
Published Values
I
R,G
R
R
R,G
R
R,G
R,G
R,G
Notes
(2)
(2)
a C
(6)
(6)
(1)
(5)
(4)
C
a C
(9)
(19)
11.005
(25) 3a
(29)
(5)
10.003
78.992
100
0.266
9.220
90.514 (31)
100
a C
P
0.20004 (5)
0.0372
99.7628
0.366 (1)
99.634
98.889 (3)
1.111 (3)
19.82 (2) 2a C
80.18 (2)
100
92.32
768
0.0001384
99.9998616
C
a
99.984426 (5) 2a C
0.015574 (5)
Best
Measurement
from a Single
Natural Source
66CAT1
56WHI1
66WAL1
2OAST1
76BAE1
58JUN1
57CRA1
69BIE1
63LEI1
73FLE1
76CLA1
7OHAG1
Reference
(Appendix A)
NBS-SRM 980*
Air*
IAEA- SLAP
IAEAVSMOW*,
NBS-RS 20
10.00
11.01
78.99
100
9.22
90. 51
0.27
100
(3)
(1)
(2)
(3)
(1)
(3)
99.762 (15)
0.038 (3)
0.200 (12)
99.63 (1)
0.37 (1)
Air
NBS-RS NSVEC*
98.90 (3)
1.10 (3)
20.0 (2)
80.0 (2)
100
92.5 (2)
7.5 (2)
0.000138 (5)
99.999862 (5)
(for air only)
99.985 (1)
0.015 (1)
(for water only)
Representative
Isotopic
Composition
NBS-RS 20*
NBS-SRM 951
JRCGEEL*,
NBS-RS LSVEC*
Air*
IAEA-V - SMOW*
IAEA-SLAP
C.E.A.
Available
Reference
Materials
(Appendix B)
TABLE OF ISOTOPIC COMPOSITIONS OF THE ELEMENTS AS DETERMINED BY MASS SPECTROMETRY
t)
35
37
36
38
40
39
40
41
40
42
43
44
46
48
45
46
47
48
49
50
50
51
Al
Si
P
5
Cl
Ar
K
Ca
Sc
Ti
V
13
14
15
16
17
18
19
20
21
22
23
32
33
34
36
31
30
28
29
27
Element
Mass
Number
Atomic
Number
92.14
4.57
- 3.01
-
-- 95.253 - 94.638
0.780 - 0.731
4.562 - 4.001
0.0199 - 0.0153
92.41
4.73
3.14
Evaluated
Range of
Published Values
G,I
G,I
,I
I
R
R
Notes
(45) 3a C
(45)
(1)
(1)
(1)
(1)
(1)
(1)
2a
(6)
(6)
S.E. C
(46) a C
(22)
(48)
(16)
(31)
0.2497
99.7503
8.24
7.44
73.71
5.43
5.18
100
96.941
0.647
0.135
2.086
0.004
0.187
93.25811 (292) 3a
0.011672 (41)
6.73022 (292)
0.337 (1) C
0.063 (1)
99.600 (1)
75.771
24.229
0.750 (7)
4.215 (4)
0.017 (2)
95.018 (4) P
100
C
92.22933 (155) 3a C
4.66982 (124)
3.10085 (74)
100
Best
Measurement
from a Single
Natural Source
66FLE1
68BEL1
5OLEL1
7 2MOOl
5ONIE1
NBS-SRM 915*
NBS-SRM 985*
Air*
(2)
(2)
(3)
0.250 (1)
99.750 (1)
8.2 (5)
7.4 (3)
73.8 (5)
5.4 (2)
5.2 (3)
100
96.941 (2)
0.647 (2)
0.135 (2)
2.086 (2)
0.004 (2)
0.187 (2)
93. 2581 (30)
0. 0117 (1)
6.7302 (30)
0.337
0.063
99.600
75.77 (5)
24.23 (5)
NBS-SRM 975*
625H12
(6)
(1)
(8)
(1)
95.02
0.75
4.21
0.02
100
92.23 (1)
4.67 (1)
3.10 (1)
TROILITE*
IAEA
C.E.A.
NBS-SRM 990*
100
Representative
Isotopic
Composition
5OMAC1
63LEI1
75BAR2
56WH11
Reference
(Appendix A)
Available
Reference
Materials
(Appendix B)
()
0'
()
N.)
C)
0
COMMISSION ON ATOMIC WEIGHTS AND ISOTOPIC ABUNDANCIES
2364
a)
•r 0
4-
c
4)
H
4)
0
U)
a)4) 0
U)0 Q
LI)
a)U) E
0
U
a)
a)a)U)
Ur1
ci k.
a) •-
•c q4a) a) a)
a)c
t') rH
'--s
'
0
N- LI) I)
00 N- N) t)
V) Q N)
LI)
00
N) t) N)
N-
—
0)
—1
tI) C r-
N) H — LI)
0
00 \0 rH I)
N)
—
'0 N)
N) N)
-1
N) —
N- t)
r-1 00
\0 0 1 00 '0
00 I)
0
00 N-
)C
4C
C)
N-
\0
N-
C)
C)
E:
0r
0
Cl)
Cl)
Cl)
Cl)
z
z
N)
00 ©
N) r
—
V) t-') i
HC
LI)
©
© N- N- '0N-
'0 ')
V)
00 LI)
C))
t)
N) N)
—
a)
—
::
Cl)
-)
)
a)a)
'0
a)
U
0
a)L)
\0
'-0
r—4
rH
-
Cl)
C/)
V)
'-0
N-
ci
N) LI) Q N-
U)
cE
a) 04)
r—l r4 rH rH
00 0
LI) C)
- LI) LI)
-VC
00
C)
C)
—
LI)rN)C)
—'--
C)
N- N)
LI) C)
r4 00
000r1tI)C)
C)C)
N) C)
C)
C)
—
N)
'0
N- C) ') C) ©
C)
00 N- r) N)
t') N- LI) t)
C)
N)
C)
N) N)
LI)
0 N)
t-)
L()
U
V)
'-'-"--"-
0
N-
N)
N-
U
k
N)
00 r r
00
0
C)
a) r d'LI) r C))
U) a) C/)
t)
N-
t')
0
a)
-H C/)
rH
0 t')
0000
V),'0
r
00 t)
C) C)
——
NtI)p
H 00 N) 00
N)
\d
00
V)
0
'00) N- 0
00N-00C)
N)
LI) N)
rH
N)
N- N)
C) C)
C)
'0
C)
t')
N) tI) '0 V)'0
N- LI) N-
LI)
C)N-N-'-0N-
t')
N) N)
C)
C)
—
U)
a)
4)
0
U)
LI) -
N- N) rH 00
N- LI)
N)
a)
a)0 C
'00)
N- C)
N)
C)
ca)
r-1
c c0
a)
U)
- C)
-H
C) N) V) LI) LI) LI) LI)
LI)
LI)
'0 N- 00
LI) LI) LI) LI)
a)
U
0
jN)
-H a)
a)
LI)
'0
N)
N)
N- 00 N)
C) '
'0
00N--00C)
N) 1
'0 t')
N- LI) C)
N) C)
C) C) rH N- '0
cx;
'0 N)
t', —
C)
00 C) rH N)
LI)
LI) '-0
o •r4
U
Z
NN)
'0 '0
- N)
z
a)
C) N-
r-l
N) LI)
N) - N) N-
— N) C)
' C)
00 '0
-- — '0
N-
LI)
C) N- N) V)
&)a)
LI) C)
v;c;
'0
LI)N)C)
4)
V)
C) C)
00
N)
'0 '0 '0
00 NC
'0 V)
- N)
V) LI)
00
00
C) LI)
C)
C)
LI)
t')
'0 N- 00 C)
'0 '0 '0 '0 N-
U
N
C) N)
LI) N)
C)N-N-'0NN)
'0
t')
00 r-l N-
C) '0 00
00 C)
'0 '0
N) 0rH N-'0
C)
C) N- N- N- NN) N)
V)
C)
C) N) t')
—
V)
N)
'0 N-
'0
N- N- N- N- N-
V)
LI)
N-
78
80
82
83
84
86
85
87
84
86
87
88
90
91
92
94
96
93
Se
Br
Kr
Rb
Sr
Y
Zr
Nb
34
35
36
37
38
39
40
41
2.9
51.7
11.23
17.4
17.57
0.58
9.99
7.14
82.75
72.24
27.86
0.36
2.29
11.58
11.55
57.14
17.44
-
-
-
-
2.79
51.12
10.8
17.1
17.38
82.29
9.75
6.94
0.55
-
-
72.14
27.76
56.90
17.24
11.44
0.341
2.223
11.49
-
-
-
-
-
-
0.877
8.932
7.640
23.497
49.655
9.399
D,G
G,R
G,D
G,D
I
Ra
-
-
0.888
9.002
7.680
23.560
49.538
9.331
Notes
(9)
(11)
(14)
(1)
(3)
(3)
(4)
(4)
(1)
(1)
(1)
(1)
p
(59) a
(15) 3o C
(26)
(14)
(46)
100
2.759 (4)
11.320 (15)
17.189 (21)
17.283 (21)
51.449
100
0.5574
9.8566
7.0015
82.5845
•
72.1654 (132) 3o C
27.8346 (132)
0.360
2.277
11.58
11.52
56.96
17.30
49. 314 (47)
50.686 (47) 3a C
0.88
8.95
7.65
23.51
49.62
9.39
Best
Measurement
from a Single
Natural Source
56WHI1
785H12
57C0L1
8OMOOl
69CAT1
73WAL1
64CAT1
48WHI1
Reference
(Appendix A)
aA range has been established which is smaller than values reported in the literature.
89
79
81
74
76
77
78
80
82
Element
Mass
Number
Atomic
Number
Evaluated
Range of
Published Values
NBS-SRM's 987*
988, 607
NBS-SRM 984*
Air*
NBS-SRM 977*
Available
Reference
Materials
(Appendix B)
(1)
(1)
(1)
(3)
(4)
(3)
100
51.45 (18)
11.32 (5)
17.19 (6)
17.28 (6)
2.76 (1)
100
0.56 (1)
9.86 (1)
7.00 (1)
82.58 (1)
72.17 (2)
27.83 (2)
0.35 (2)
2.25 (2)
11.6 (1)
11.5 (1)
57.0 (3)
17.3 (2)
50.69 (5)
49.31 (5)
23.5
49.6
9.4
7.6
0.9
9.0
Representative
Isotopic
Composition
0\
U,
C',)
CD
rP
0
103
102
107
109
106
108
110
111
112
113
Mo
Tc
Ru
Rh
Pd
Ag
Cd
In
42
43
44
45
46
47
48
49
113
115
114
116
4.33
95.84
18.67
104
104
105
106
108
110
31.7
5.57
1.91
12.77
12.69
17.1
15.05
9.35
15.93
16.71
9.6
24.42
9.63
96
98
99
100
101
102
92
94
95
96
97
98
100
Element
Mass
Number
Atomic
Number
9.48
24.00
9.60
-
-
---
4.16
95.67
5.47
1.84
12.7
12.56
- 17.01
- 31.52
- 18.5
-
-
- 14.74
- 9.11
- 15.78
- 16.56
Evaluated
Range of
Published Values
D,G
G,I
I
G,I
D,G
D,G
Notes
(159)
(96)
(1)
(2)
(2)
(1)
(3)
(3)
(1)
a
(96)
(26) 3a C
(5)
(2)
(6)
(6)
(1)
(1)
(1) 2a C
(1)
(1)
(1)
(1)
(1)
4.33 (4)
95.67 (4)
1.25
0.894
12.51
12.81
24.13
12.22
28.71
7.47
48.170 (26)
51.830
27.33
26.46
11.72
22.33
1.020 (8) 2o C
11.14 (5)
100
5.52
1.86
12.74
12.60
17.05
31.57
18.66
9.6335
24.1329 (241)
9.5551
16.6756 (167)
15. 9201
14.8362 (148) 2a
9.2466 (92)
Best
Measurement
from a Single
Natural Source
56WH11
75R051
95.7
4.3
7.47
28.72
12.51
12.81
24.13
12.22
1.25
0.89
(2)
(2)
(2)
(1)
(2)
(2)
(2)
(2)
(2)
(2)
(3)
(3)
51.83
48.17
(1)
(1)
(1)
(2)
(2)
625H11
100
17.0
31.6
18.7
12.7
12.6
1.020 (12)
11.14 (8)
22.33 (8)
27.33 (3)
26.46 (9)
11.72 (9)
NBS-SRM 978*
(2)
(1)
(2)
(2)
(1)
(3)
(1)
5.52 (5)
1.88 (5)
14.84
9.25
15.92
16.68
9.55
24.13
9.63
Representative
Isotopic
Composition
78SHI1
63LEI1
76DEV1
74MOOl
Reference
(Appendix A)
Available
Reference
Materials
(Appendix B)
H
t.i
H
0
H
0
H
C'
C'
Element
Sn
Sb
Te
I
Xe
Cs
Atomic
Number
50
51
52
53
54
55
133
131
132
134
136
129
130
128
124
126
127
125
126
128
130
124
123
122
120
121
123
124
122
112
114
115
116
117
118
119
120
Mass
Number
0.102
0.098
1.93
26.51
4.07
21.24
27.12
10.54
8.98
24.31
8.68
33.11
4.78
6.11
7. 767
14.78
0. 681
0. 376
1.017
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
8.87
21.04
26.88
10.43
3.68
26.24
1.91
0.095
0.088
0.61
0.33
14.07
7.51
23.84
8.45
32.34
4.559
5.626
0.90
Evaluated
Range of
Published Values
D,G
G,I
57.25 (3)
42.75 (3)
100
0.096
0.090
1.919
26.44
4.08
21.18
26.89
10.44
8.87
100
2.603
0.908
4.816
7.139
18.952
31.687
33.799
(7)
(3)
(1)
(3)
(3)
(5)
(7)
(7)
(1)
(1)
(4)
(8)
(1)
(5)
(7)
(2)
(1)
0.0960
(8)
(3)
(3)
(3)
(8)
(3)
(5)
(3)
X
(3)
(3)
1.01
0.67
0.38
14.76
7.75
24.30
8.55
32.38
4.56
5.64
D,G
Notes
P
2o
Best
Measurement
from a Single
Natural Source
56WH Il
50N1E2
49LEL1
78SMI1
48WHI1
65LAE1
Reference
(Appendix A)
Air*
Available
Reference
Materials
(Appendix B)
(1)
(1)
(1)
(3)
(6)
(1)
(1)
(2)
(2)
100
8.9
(1)
21.2 (4)
26.9 (5)
10.4 (2)
4.1 (1)
26.4
0.10
0.09
1.91
100
18.95
31.69
33.80
7.14
0.908 (3)
4.816 (8)
2.60
0.096 (2)
57.3 (9)
42.7 (9)
1.0 (2)
0.7 (2)
0.4 (2)
14.7 (3)
7.7 (2)
24.3 (4)
8.6 (2)
32.4 (4)
4.6 (2)
5.6 (2)
Representative
Isotopic
Comp osi t ion
(')
t-,)
'-0
27.3
12.32
23.97
8.35
17.35
5.78
5.69
141
142
143
144
145
146
148
150
---
144
Ce
Pr
Nd
Pm
Sm
Eu
58
59
60
61
62
63
47.86
52.25
26.90
22.88
7.47
3.16
15.10
11.35
13.96
0.190
0.250
88.449
11.07
2.87
47.75
52.14
26.55
22.43
7.36
14.87
- 11.22
- 13.82
-
-
26.80
- 12.12
- 23.795
- 8.23
- 17.06
- 5.66
- 5.53
-
-
b
D,G
D,G
D,G
D,G
G
G,I
Notes
(2)
(2)
(3)
(2)
(4)
(4)
(7)
(3)
(4)
(2)
47.77 (20)
52.23 (20)
3.12
15.10
11.30
13.86
7.38
26.65
22.59
17.188
5.755
5.635
8.293
27.157
12.177
23.795
88.475 (8)
11.081 (7)
0.1904
0.2536
99.911
0.089 (2)
2.417
6.592
7.853
11.232
71.699
0.1058
0.1012
bThe only two available measurements give identical values.
151
153
147
148
149
150
152
154
139
138
0.195
0.265
88.48
11.098
136
138
140
142
La
57
138
137
135
136
130
132
134
Ba
56
Mass
Number
Element
Atomic
Number
Evaluated
Range of
Published Values
2o
S.E. C
Best
Measurement
from a Single
Natural Source
48HES1
75LUG1
74BAR1
7 6NAK1
57C0L1
62UME1
56WHI1
471NG2
69EUG1
Reference
(Appendix A)
Available
Reference
Materials
(Appendix B)
(.1)
(2)
(2)
(1)
(2)
(1)
(1)
(1)
(3)
(7)
(2)
(3)
(2)
(2)
(7)
47.8 (5)
52.2 (5)
26.6 (2)
22.6 (2)
7.4
3.1
15.1
11.3
13.9
27.16
12.18
23.80
8.29
17.19
5.75
5.63
100
88.48 (10)
11.08 (10)
0.25
99.91
0.09
0.106 (2)
0.101 (2)
2.417 (27)
6.592 (18)
7.854 (39)
11.23 (4)
71.70 (7)
Representative
Isotopic
Composition
C'
cx:
Ni
152
156
158
160
161
162
163
164
165
162
164
169
168
170
171
Element
Gd
Tb
Dy
Ho
Er
Tm
Yb
Lu
64.
65
66
67
68
69
70
71
175
176
174
176
173
172
170
167
168
166
159
155
156
157
158
160
154
Mass
Number
Atomic
Number
27.07
15.04
-
-
22. 94
-
-
-
-
-
-
-
-
-
0.154
1.60
33.41
0.064
0.105
2.36
19.0
25.53
24.97
28.47
0.205
2.23
15.1
20.67
15.73
24.96
22.01
33.36
22.82
27.02
14.88
1.56
0.136
0.0524
0.0902
2.294
18.73
25.36
24.9
28.1
14.68
20.36
15.64
24.5
21.6
2.1
0.20
Evaluated
Range of
Published Values
G,I
G,I
D,G
D,G
D,G
Notes
(2)
(5)
(5)
7.39-3
2.607
(20)
(9)
(10)
(6)
(1)
(3)
(9)
0.136
3.063
14.334
21.879
16.122
31.768
12.698
100
14.88 (20)
22.94
(20)
27.07 (30)
2
2 S.E.
0.136 (3) P
1.56 (3)
33.41 (30)
100
28.1
19.0 (1)
25.5 (2)
24.9 (2)
(15)
(21)
(16)
(25)
(22)
(2)
(1)
0.057 (1)
0.100 (1)
2.35 (2)
100
0.20
2.15
14.78
20.59
15.71
24.78
21.79
Best
Measurement
from a Single
Natural Source
76MCCI
77MCC1
57COL1
5OHAY1
57COL1
57C0L1
57C0L1
48HE51
Reference
(Appendix A)
Available
Reference
Materials
(Appendix B)
(2)
(4)
(4)
(4)
(3)
(1)
(4)
(5)
(4)
(6)
(6)
(1)
(6)
(4)
(.2)
(2)
97.39
2.61
0.14 (1)
3.06 (3)
14.3 (1)
21.9 (1)
16.1 (1)
31.8 (2)
12.7 (1)
100
14.9
33.4
(6)
22.9 (4)
27.1 (6)
0.14
1.56
100
19.0
25.5
24.9
28.1
0.06 (1)
0.10 (1)
2 . 34 (4)
100
24.8
21.8
15.7
20.6
2.1
14.8
0.20
Representative
Isotopic
Composition
f%J
Cl)
S
CD
S
CD
CD
CD
180
180
182
183
184
186
185
Hf
Ta
W
Re
Os
Ir
Pt
Au
72
73
74
75
76
77
78
79
197
194
195
196
198
190
192
191
193
192
190
189
188
186
187
184
187
181
174
176
177
178
179
180
Element
Mass
Number
Atomic
Number
0.0127
0.78
32.9
33.8
25.4
7.23
0.02
1.67
1.67
13.27
16.21
26.42
41.21
26.41
14.43
30.68
28.85
0.16
0.0123
99.9883
0.199
5.23
18.56
27.23
13.78
35.44
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
-
7.19
32.8
33.7
25.2
0.012
0.78
0.018
1.59
1.60
13.15
16.08
26.15
40.96
0.126
26.09
14.24
30.63
28.38
0.0117
99.9877
0.163
5.15
18.39
27.08
13.73
35.07
Evaluated
Range of
Published Values
D
X
D,G
I
D
D
D
Notes
Best
(2)
(6)
100
32.9 (1)
33.8 (1)
25.2 (1)
7.19 (4)
0.0127 (5)
0.78 (1)
37.3
62.7
63LE11
56WHI1
54BAL1
3 7N I El
0.018 (2) P
1.59 (5)
1.64 (5)
13.27 (12)
16.14 (14)
26.38 (20)
40.96 (14)
48VIHI1
7 3CRA1
(3)
(1)
(3)
(3)
56WH11
56WH11
Reference
(Appendix A)
37.398 (16) 3o C
62.602 (16)
0.126
26.31
14.28
30.64
28.64
(3)
(3)
(10)
(5)
(10)
(2)
(6)
0.0123
99.9877
0.163
5.21
18.56
27.10
13.75
35.22
Natural Source
Me as ur eme nt
from a Single
NBS-SRM 989*
Available
Reference
Materials
(Appendix B)
(2)
(2)
(3)
(3)
(3)
100
7.2
25.3
33.8
32.9
(2)
(5)
(5)
(5)
0.010 (3)
0.79 (5)
62.7
37.3
41.0
0.020 (4)
1.58 (10)
1.6 (1)
13.3 (2)
16.1 (3)
26.4 (4)
37.40
62.60
0.10 (3)
26.3 (2)
14.3 (1)
30.7 (2)
28.6 (2)
0.012 (2)
99.988 (2)
0.2 (1)
5.2 (1)
18.6 (3)
27.1 (5)
13.7 (3)
35.2 (5)
Representative
Isotopic
Composition
0
Hg
Tl
Pb
Bi
Po
At
Rn
Fr
Ra
Ac
Tb
Pa
U
Np
80
81
82
83
84
85
86
87
88
89
90
91
92
93
237
234
235
238
232
209
204
206
207
208
203
205
196
198
199
200
201
202
204
Mass
Number
0.16
0.0059
0.7202
99.2752
27.48
23.65
56.21
1.65
10.12
17.01
23.21
13.27
29.81
6.85
-
-
-
-
-
-
0.0050
0.7198
99.2739
1.04
20.84
17.62
51.28
6.69
0.147
10.02
16.83
23.07
13.12
29.64
R,Ga
R,G
D
Notes
(7)
(12)
(9)
(10) a
(10)
(9) 3a C
(9)
0.0054
0.7200
99.2746
100
100
C
1.4245 (12) 3a C
24. 1447 (57)
22.0827 (27)
52. 3481 (86)
29.524
70.476
(15)
6.79 (5)
29.64
0.156
10.12
16.99
23.07
13.27
Best
Measurement
from a Single
Natural Source
76C0W1
57S'IIl
36DEM1
63LEI1
68CAT1
8ODUN1
55D1B1
Reference
(Appendix A)
range has been established which is smaller than values reported in the literature.
Representative isotopic composition is for most but not all commercial samples.
Element
Atomic
Number
Evaluated
Range of
Published Values
C.E.A.
U0002_U970*
NBS-SRM' 5
NBS-SRM 981*
NBS-SRM 997*
Available
Reference
Materials
(Appendix B)
(5)
(5)
(6)
(4)
(8)
(3)
0.005
0.720
99.275
100
100
c
(1)
(1)
(2)
52.4 (1)
24.1 (1)
22.1 (1)
1.4 (1)
29.524 (18)
70.476 (18)
10.1
17.0
23.1
13.2
29.6
6.8
0.2 (1)
Representative
Isotopic
Composition
N)
CD
rt
2372
COMMISSION ON ATOMIC WEIGHTS AND ISOTOPIC ABUNDANCIES
Appendix A
References
2OAST1 F. W. Aston, Phil. Mag. 40, 628 (1920).
The Mass Spectra of Chemical Elements.
36DEM1 A. J. Dempster, Nature 136, 120 (1936).
Atomic Masses of Uranium and Thorium.
37NIE1 A. 0. Nier, Phys. Rev. 52, 885 (1937).
The Isotopic Constitution of Osmium.
471NG2 M. C. Inghram, R. G. Hayden, and D. C. Hess, Jr., Phys. Rev. 72,
967 (1947).
The Isotopic Composition of Lanthanum and Cesium.
47VAL1 G. E. Valley and H. H. Anderson, J. Amer. Chem. Soc., 69,
— 1871
(1947) .
A Comparison of the Abundance Ratios of the Isotopes of Terrestrial
and Meteoritic Iron.
48HE51 D. C. Hess, Jr., Phys. Rev. 74, 773 (1948).
The Isotopic Constitution of Erbium, Gadolinium, and Terbium.
48WHI1 J. R. White and A. E. Cameron, Phys. Rev. 74, 991 (1948).
The Natural Abundance of the Isotopes of Stable Elements.
49LEI1 W. T. Leland, Phys. Rev. 76, 992 (1950).
On the Abundance of 1291, 118TE and 19OPT.
5OHAY1 R. J. Hayden, D. C. Hess, Jr., and M. G. Inghram, Phys. Rev. 77,
299 (1950)
The Isotopic Constitution of Erbium and Lutecium.
5OLEI1 W. T. Leland, Phys. Rev. 77, 634 (1950).
The Isotopic Composition of Scandium, Gadolinium and Dysprosium.
5OMAC1 J. MacNamara and H. G. Thode, Phys. Rev. 78, 307 (1950).
Comparison of the Isotopic Constitution ofTerrestrial and
Meteoritic Sulphur.
5ONIE1 A. 0. Nier, Phys. Rev. 77, 789 (1950).
A Redetermination of the Relative Abundances of the Isotopes of
Carbon, Nitrogen, Oxygen, Argon, and Potassium.
50N1E2 A. 0. Nier, Phys. Rev. 79, 450 (1950).
A Redetermination of the Relative Abundances of the Isotopes of
Neon, Krypton, Rubidium, Xenon, and Mercury.
53REY1 J. H. Reynolds, Phys. Rev. 90, 1047 (1953).
The Isotopic Constitution of Silicon, Germanium, and Hafnium.
54BAL1 R. Baldock, U.S. Atomic Energy Commission, Rept. ORNL 1719 (1954).
ORNL Status and Progress Report, April 1954.
55DIB1 V. H. Dibeler, Anal. Chem. 27, 1958 (1955).
Isotope Analysis Using Dimethylmercury.
56WH11 F. A. White, T. L. Collins, Jr., and F. M. Rourke, Phys. Rev. 101,
1786 (1956).
Search for Possible Naturally Occurring Isotopes of Low Abundance.
57C0L1 T. L. Collins, Jr., F. M. Rourke, and F. A. White, Phys. Rev. 105,
196 (1957)
Mass Spectrometric Investigation of the Rare Earth Elements for
the Existence of New Stable Isotopes.
57CRA1 H. Craig, Geochim. Cosmochim. Acta 12, 133 (1957).
Isotopic Standards for Carbon and Oxygen and Correction Factors
for Mass Spectrometric Analysis of Carbon Dioxide.
Atomic weights of the elements 1979
2373
57SMI1 R. F. Smith and J. M. Jackson, U.S.A.E.C. KY-58l (1957).
Variations in U238 Concentration of Natural Uranium.
58JUN1 G. Junk and H. J. Svec, Geochim. Cosmochim. Acta 14, 234 (1958).
The Absolute Abundance of the Nitrogen Isotopes in the Atmosphere
and Compressed Gas from Various Sources.
625H11 IV. R. Shields, E. L. Garner, and V. H. Dibeler, J. Res. Nat. Bur.
Stand. 66A, 1 (1962).
Absolute Isotopic Abundance of Terrestrial Silver.
625HI2 W. R. Shields, T. J. Murphy, E. L. Garner, and V. H. Dibeler,
J. Am. Chem. Soc. 84, 1519 (1962).
Absolute Istopic Abundance Ratios and the Atomic Weight of Chlorine.
62UME1 S. Umemoto, J. Geophys. Res. 67, 375 (1962).
Isotopic Composition of Barium and Cerium in Stone meteorites.
63LE11 F. D. Leipziger, Appl. Spec. 17, 158 (1963).
Some New Upper Limits of Isotopic Abundance by Mass Spectrometry.
64CAT1 E. J. Catanzaro, T. J. Murphy, E. L. Garner and W. R. Shields,
J. Res. Nat. Bur. Stand. 68A, 593 (1964).
Absolute Isotopic Abundance Ratio and the Atomic Weight of Bromine.
64S1111 W. R. Shields, T. J. Murphy, and E. L. Garner, J. Res. Nat. Bur.
Stand. 68A, 589 (1964)
Absolute Isotopic Abundance Ratios and the Atomic Weight of a
Reference Sample of Copper.
65LAE1 J. R. DeLaeter and P. M. Jeffery, J. Geo. Phys. Res., 70, 2895 (1965).
The Isotopic Composition of Terrestrial and Meteoritic Tin.
66CAT1 E. J. Catanzaro, T. J. Murphy, E. L. Garner, and W. R. Shields,
J. Res. Nat. Bur. Stand. 70A, 453 (1966).
Absolute Isotopic Abundance Ratios and the Atomic Weight of Magnesium.
66FLE1 G. D. Flesch, J. Capellen, and H. J. Svec, Adv. Mass Spec. III, 571,
1966, Leiden and Son, London.
The Abundance of the Vanadium Isotopes from Sources of Geochemical
Interest.
66SH11 W. R. Shields, T. J. Murphy, E. J. Catanzaro, and E. L. Garner,
J. Res. Nat. Bur. Stand. 7YA, 193 (1966).
Absolute Isotopic Abundance Ratios and the Atomic Weight of a
Reference Sample of Chromium.
66WAL1 J. R. Walton and A. E. Cameron, Z. Naturforsch. 2lA, 115 (1966).
The Isotopic Composition of Atmospheric Neon.
68BEL1 H. A. Belsheim, Iowa. State Univ., Thesis-217 (1968).
Absolute Abundance of the Titanium Isotopes in Nature.
68CAT1 E. J. Catanzaro, T. J. Murphy, W. R. Shields, and E. L. Garner,
J. Res. Nat. Bur. Stand. 72A, 261 (1968)
Absolute Isotopic Abundance Ratios of Common, Equal-Atom, and
Radiogenic Lead Isotopic Standards.
69B1E1 P. J. De Bievre and G. H. Debus, Int. J. Mass Spectrom. Ion Phys.
2, 15 (1969).
Absolute Isotope Ratio Determination of a Natural Boron Standard.
69CAT1 E. J. Catanzaro, T. J. Murphy, E. L. Garner, and W. R. Shields,
J. Res. Nat. Bur. Stand. 73A, 511 (1969).
Absolute Isotopic Abundance Ratios and the Atomic Weight of
Terrestrial Rubidium.
69EUG1 0. Eugster, F. Tera, and G. J. Wasserburg, J. Geophys. Res. 74,
3897 (1969)
Isotopic Analyses of Barium in Meteorites and in Terrestrial
Sam-es.
2374
COMMISSION ON ATOMIC WEIGHTS AND ISOTOPIC ABUNDANCIES
7OHAG1 R. Hagernann, G. Nief, and ]. Roth, Tellus 22, 712 (1970).
Absolute Isotopic Scale for Deuterium Analysis of Natural Waters,
Absolute D/H Ratio for SMOW.
72M001 L. J. Moore and L. A. Machlan, Anal. Chem. 44, 2291 (1972).
High Accuracy Determination of Calcium in Blood Serum by Isotope
Dilution Mass Spectrornetry.
72ROS1 K. J. R. Rosman, Geochim. Cosmochim. Acta 36, 801 (1972).
A Survey of the Isotopic and Elemental Abundance of Zinc.
73BAR1 I. L. Barnes, E. L. Garner, J. W. Gramlich, L. A. Machlan,
J. R. Moody, L. J. Moore, T. J. Murphy, and W. R. Shields, Proc.
Fourth Lunar Sci. Conf., Geochim. Cosmochim. Acta Suppl. 4, 2,
1197 (1973)
Isotopic Abundance Ratios and Concentrations of Selected Elements
in Some Apollo 15 and Apollo 16 Samples.
J.
73FLE1 G. D. Flesch, A. R. Anderson, Jr., and H. J. Svec, mt.
Mass
Spectrom. Ion Phys. 12, 265 (1973).
A Secondary Isotopic Standard for Li-6/Li-7 Determinations.
73GRA1 J. W. Gramlich, T. J. Murphy, E. L. Garner, and W. R. Shields, J.
Res. Nat. Bur. Stand. 77A, 691 (1973).
Absolute Isotopic Abundance Ratio and Atomic Weight of a Reference
Sample of Rhenium.
73WAL1 J. R. Walton et. al., Int. J. Mass Spectrom. Ion Phys., 12, 439
(1973)
Determination of the Abundance of Krypton in the Earth's Atmosphere
by Isotope Dilution Mass Spectrometry.
74BAR1 I. L. Barnes, Private Communication, August 1974.
74MOOl L. J. Moore, L. A. Machlan, W. R. Shields, and E. L. Garner, Anal.
Chem. 46, 8 (1974).
Internal Normalization Techniques for High Accuracy Isotope Dilution
Analyses - Application to Molybdenum and Nickel in Standard
Reference Materials.
75BAR2 I. L. Barnes, L. J. Moore, L. A. Machlan, T. J. Murphy, and
W. R. Shields, J. Res. Nat. Bur. Stand. 79A, 727 (1975).
Absolute Isotopic Abundance Ratios and Atomic Weight of a Reference
Sample of Silicon.
75GAR1 E. L. Garner, T. J. Murphy, J. W. Gramlich, P. J. Paulsen, and
I. L. Barnes, J. Res. Nat. Bur. Stand. 79A, 713 (1975).
Absolute Abundance Ratios and the Atomic Weight of a Reference
Sample of Potassium.
75LUG1 G. W. Lugmair, N. B. Scheinin, and K. Marti, Proc. Lunar Sci. Conf.,
6th, Geochim. Cosinochim. Acta Suppl. 6, 2, 1419 (1975).
Sm-Nd Age and History of Apollo 17 Basalt 75075: Evidence for
Early Differentiation of the Lunar Exterior.
75ROS1 K. J. R. Rosman and J. R. DeLaeter, Int. J. Mass Spectrom. Ion
Phys., 16, 385 (1975).
The Isotopic Composition of Cadmium in Terrestrial Minerals.
76BAE1 P. Baertsch, Earth Planet. Sci. Lett., 31 341 (1976).
Absolute l& Content of Standard Mean Ocean Water.
76CLA1 W. B. Clarke, et al., Tnt. J. Appl. Radiat. Isotopes, 27, 515
(1976).
Determination of Tritium by Ilass Spectrometric Measurement of 3He.
76COW1 G. A. Cowan and H. H. Adler, Geochim. Cosmochim. Acta, 40, 1487
(1976)
The Variability of the Natural Abundance of 235U.
Atomic weights of the elements 1979
76DEV1 C. Devillers et. al., Proc. 7th mt. Mass Spectromet. Conf.
Florence, (1976).
Mass Spectrometric Investigations on Ruthenium Isotopic Abundances.
76LAE1 J. R. DeLaeter and K. J. R. Rosman, mt.
Phys. 21, 403 (1976).
The Atomic Weight of Gallium.
J.
Mass Spectrom. Ion
76MCC1 McCulloch et. al., Earth Planet. Sci. Lett. 28, 308 (1976).
The Isotopic Composition and Elemental Abundance of Lutetium in
Meteorites and Terrestrial Samples and the '76Lu Cosmochronometer.
76NAK1 N. Nakamura et. al., Proc. Lunar Sci. Conf. 7, 2, 2309 (1976).
4.4 b.y. -Old Clast in Boulder 7, Apollo 17 — A Comprehensive
Chronological Study by U-Pb, Rb-Sr, and Sm-Nd Methods.
77MCC1 M. T. McCulloch, K. J. R. Rosman, and J. R. DeLaeter, Geochim.
Cosmochim. Acta. 41, 1703 (1977).
The Isotopic and Elemental Abundance of Ytterbium in Meteorites
and Terrestrial Samples.
78SHI1 M. Shima, C. E. Rees, and H. G. Thode, Can. J. Phys., 56, 1333
(1978)
The Isotopic Composition and Atomic Weight of Palladium.
78SHI2 M. Shima, Tnt. J. Mass Spectrom. Ion Physics, 28, 129 (1978).
Isotopic Composition of Zirconium.
78SMI1 C. L. Smith, K. J. R. Rosman, and J. R. DeLaeter, Tnt. J. Mass
Spectrom. Ion Phys., 28, 7 (1978).
The Isotopic Composition of Tellurium.
8ODUN1 L. P. Dunstan, J. W. Gramlich, I. L. Barnes, and W. C. Purdy, J.
Res. Nat. Bur. Stand., 85, No. 1, 1 (1980).
Absolute Isotopic Abundance and the Atomic Weight of a Reference
Sample of Thallium.
8OMOOl L. J. Iloore, T. J. Murphy, and I. L. Barnes, J. Res. Nat. Bur.
Stand., to be published (1980).
The Absolute Abundance Ratios and Isotopic Composition of a
Terrestrial Sample of Strontium.
2375
2376
COMMISSION ON ATOMIC WEIGHTS AND ISOTOPIC ABUNDANCIES
Appendix B
Sources ofReference Materials
I .A.E.A.
Samples such as V-SMOW, SLAP, and SLAG may be obtained from:
International Atomic Energy Agency
Section of Hydrology
A-lOll Vienna, Kaerntnerring, (Austria)
TROILITE
Canon Diablo Troilite may be obtained from:
Mr. Glenn I. Huss
Director, American Meteorite Laboratory
P.O. Box 2098
Denver, Colorado 80201 (U.S.A.)
NBS-SRM' s
NBS Standard Reference Materials may be purchased through:
Office of Standard Reference Materials
National Bureau of Standards
B311 Chemistry Building
Washington, D. C.
20234 (U.S.A.)
JRC-GEEL
Reference Materials may be obtained through:
Dr. Paul De Bievre
European Commission
Central Bureau for Nuclear Measurements
B-2440 Geel, (Belgium)
NBS-RS (Reference Samples)
Samples may be obtained through:
Chief, Inorganic Analytical Research Division
National Bureau of Standards
A219 Chemistry Building
Washington, D. C. 20234 (U.S.A.)
NOTE: Samples of N and Li previously available from
Professor H. J. Svec have been sent to NBS for distribution.
C.E.A.
Standards may be obtained through:
Dr. R. Hagemann
Centre d'Etudes de Saclay
B.P. n°2 - 91190 Gif-sur-Yvette (France)
1
12
13
16
17
18
0
3
4
2
C
He
H
Element
Stable
Mass
Numbers
TABLE 4.
H
from +20 to
Up to 1% pure
component
7,,,
(2) Bulk
10
518 >
(1) Minerals
Allende meteorite
(minerals)
S18 up
Lunar rocks
16
,
Standard Mean
Ocean Water
to 6.5
18o
= 0 for
—
+49
,
618 as 'large as
—30
'3C
wind carbon
Nucleosynthesis
anomaly + mass
fractionation
Mass fractionation
isotope exchange
equilibria
tempe ratures
Mass fractionation
at different
Mass fractionation
in surface processes
Solar
and
spallation
reaction
4OO
Solar wind
synthesis?
Varying nucleo—
4x106
5x1O4
3He/4He =
1
3He/4He " 4.4x10'4
times earth ratio
=
Process
Bombardment by
solar wind
H
samples.
Probable
6D as low as —846
2x106 and less
than 2x104
D greater than
Range of Value
Lunar soil,
surface
Lunar soil
Lunar surface
material
Lunar soil
Optical measure—
ments on inter—
stellar matter
Source of Sample
Representative
Value
1979 SAIC
Examples of variations in isotopic composition for selected elements in non—terrestrial
77 Cla
74 Onu
77 Cla
70 Onu
73 Tay
73 Eps
71 Meg
73 Eps
73 Bla
Ref.
—4
—4
a
5
28
29
Si
size <
Lunar
Lunar
32
soil bulk
511
soil grain
Meteorite minerals
33
34
36
30
Lunar soil surface
inclusions
Allende
meteorite
24
25
26
Mg
21
22
radioactive
S34 as high as
+11 cx,
as high as
,
Mass fractionation
Mass fractionation
+20
34
Mass fractionation
decay of 26Al
Extinct
reactions
Cosmic ray
spallation
Mass fractionation
—
.27%
9.22%
90.51%
Spallation component
Early solar wind
nitrogen
wind nitrogen
Present day solar
fractionation
Nucleosynthesis
anomaly + mass
Process
Probable
S30Si as high as
+18
up to +400
t30Si as high as
26Mg
to 30.88%
33.05 to 32.89%
36.64 to 36.72%
Pure '5N
Lunar step heating
30.3
SlSN up to —105
S'5N up to +120
Up to 5% pure 16o
Range of Value
Lunar drill core
Lunar soil surface
(3) Mineral
separates
Source of Sample
Sodium rich
minerals in
meteorites
15
14
18
17
16
Numbers
20
Ne
N
0
Element
Mass
1979 SAIC
Representative
Value (in atom %)
Ref.
76 Tho
76 Tho
73 Tay
73 Tay
79 Bra
75 Smi
77 Bec
77 Bec
77 Bec
77 Cla
Examples of variations in isotopic composition for selected elements in non—terrestrial samples. (cont'd)
Stable
TABLE 4.
Meteorite
Tieschitz
Meteorites
124
126
128
Xe
Allende meteorite
(Hibonite)
Mars Atmosphere
Allende meteorite
Carbonaceous
meteorites
106
108
110
111
112
113
114
115
116
43
44
46
48
42
40
38
40
36
33
34
33
32
Source of Sample
Cd
Ca
Ar
S
Element
0,
0/
per mass
unit
'29Xe/'32Xe as large
as 12 times atmos—
pheric ratio.
from 110 to 116
?
2.3 ? per mass
unit for isotopes
Non—lunar effect
2
per mass unit
favoring heavy
isotopes
7.5
Isotope shift
uniform
0.030%
0.006%
99.96%
S33S up to 1
as high as +2.5
and as low as —1.7 0/
Range of Value
0/
0.06%
99.6%
0.33%
Representative
Value (in atom %)
1979 SAIC
Radiogenic, from the
decay of traRped
129i to Xe12'
Mass fractionation
Nuclear effect
Mass fractionation
fractionation?
Atmospheric mass
anomaly
Nucleosynthesis
Mass fractionation
Process
Probable
Ref.
74 Rey
78 Ros
79 Lee
79 Lee
76 Nie
77 Ree
65 Hul
Examples of variations in isotopic composition for selected elements in non—terrestrial samples. (cont'd)
Stable
Mass
Numbers
TABLE 4.
r)
Eps
Onu
Meg
Bia
Hul
77
77
77
78
78
79
74
75
76
76
77
Tho
Ard
Bec
Cia
Ree
Bra
Ros
Lee
Nie
Sini
Rey
Tay
74 Ony
65
70
71
73
73
73
234
235
238
U
Meteorites
Meteorites
Source of Sample
from —2
to —225 7
7
77 Ard
74 Rey
Ref.
Institute,
tion of 247Cf with
uranium and subsequent
decay of 247Cf to
Nucleosynthesis or
chemical fractiona—
components
238U
Fission product
Process
Probable
For
isotopes.
example, '36Xe excess
as large as 150%.
Value
Excess of heavy
Range of Value
J.R. Huistom and H.G. Thode, J. Geophys. Res., 70, 3475 (1965).
N. Onuma, R.N. Clayton and T.K. Mayeda, Proc. Apollo ilLun. Sci. Conf., 2, 1429 (1970).
G.H. Megrue, J. Geophys. Res., 76, 4956 (1971).
J.H. Black and A. Delgarno, Astrophy. .J. 184, L10l, 1973.
S. Epstein and H.P Taylor, Jr., Proc Lun. Sci. Conf., 4, 1559 (1973).
H.P. Taylor and S. Epstein, Proc. Lun. Sci. Conf., 4, 1657 (1973).
N. Onuma, R.N. Clayton and T.K. Mayeda, Geochim. Cosmochim, Acta, 38, 189 (1974).
J.H. Reynolds, Proc. Soviet—American Conf. on Cosmochemistry of Moon and Planets, Moscow, June 1974.
S.P. Smith and J.C. Huneke, Earth Planet. Sci. Lett., 27, 191 (1975).
A.O. Nier, M.B. McElroy and Y.L. Yung, Science, 194, 68 (1976).
H.G. Thode and C.E. Rees, Proc. Lun. Sci. Conf., 7, 459 (1976).
J.W. Arden, Nature, 269, 788 (1977).
R.H. Becker and R.N. Clayton, Proc. Lunar and Planet. Sci Conf., 8, 3685 (1977).
R.N. Clayton, N. Onuma, L. Grossman and T.K. Mayeda, Earth Planet. Sci. Lett., 34, 209 (1977).
C.E. Rees and H.G. Thode, Geochim. Cosmochim. Acta, 41, 1679 (1977).
J.G. Bradley, J.C. Huneke and G.J. Wasserburg, J. Geophys. Res., 83, 1244 (1978).
K.J.R. Rosman and J.R. DeLaeter, J. Geophys. Res., 83, 1279 (1978).
T.M. Lee, W.A. Russell and G.J. Wasserburg, in Lunar and Planetary Science X, The Lunar and Planetary
Houston, 713 (1979).
REFERENCES TO TABLE 4
129
130
131
132
134
Xe
Element
Mass
Numbers
1979 SAIC
Representative
Examples of variations in isotopic composition for selected elements in non—terrestrial samples. (cont'd)
Stable
TABLE 4.
Atomic weights of the elements 1979
2381
different parts of the universe. Other nuclear processes such as radioactive decay, nuclear
fission or fusion and nuclear reactions induced by cosmic ray bombardment or natural radioac—
tivity can enhance or deplete specific isotopes in a sample.
Finally, solar wind implantation is an example of a third series of processes that can mod—
ify isotopic abundances. Bulk currents of particles with modified isotope ratios which orig—
mate in other parts of the universe can be implanted in samples by collision sometimes in
sufficient quantities to measurably modify the isotopic abundance of these elements in the
samples.
It is often the case that more than one of these processes can occur in a given sample. For
example, the measurements of magnesium isotope ratios in Allende meteorite samples by
Wasserburg et al. (Ref. 63) have led to the conclusion that both mass fractionation and an
unknown nuclear process have contributed to the isotopic abundance variations.
Table 4 lists a series of examples of measurements of isotopic abundance for various ele—
ments in indicated non—terrestrial samples in which variation in isotopic composition from
terrestrial values is reported. We have chosen these table entries to illustrate the range
of variation as well as the variety of processes which can produce them.
Isotopic abundance variations are reported in several different but related ways. In some
cases a numerical isotope ratio such as D/H
1.5 x i04 is employed. For multi—isotopic el—
ements the percentage abundance of each isotope is sometimes listed and can be compared di—
rectly to similar abundance information for terrestrial material. In many cases, he variation given is reported as a 'del' value which is expressed in parts per thousand (fix))
where
S(A) y
= (measured isotope ratio A/B — (standard isotope ratio A/B) x 1000
standard isotope ratio A/B
One of these three quantities is employed in Table 4 to illustrate isotopic variation for a
given element and sample. Where comparison is required (i.e., for isotope ratio and isotopic abundance data), the 1979 SAIC representative value is listed for comparison.
Although this discussion has concentrated on variations of isotope ratios in non—terrestrial
samples, in a number of cases isotopic abundances are the same in non—terrestrial and terrestrial samples. For example, agreement has been reported for lutetium (Ref. 64) and telluri—
inn (Ref. 65) in meteoritic samples, and for magnesium, calcium, nickel, chromium, rubidium
and uranium (Ref. 28) and potassium, strontium, lead and thorium (Ref. 66) in various lunar
samples.
RELATIVE ATOMIC MASSES AND HALF-LIVES OF SELECTED HADIONUCLIDES
For many years the Commission on Atomic Weights has included in its Reports tables of relative atomic masses of selected nuclides and half—lives of some radionuclides, although it
has no prime responsibility for the dissemination of such values. No attempt has,
therefore, been made to state these values at the best precision possible or to make them
any more complete than is needed to enable users to calculate the atomic weights of materials of abnormal or changing isotopic composition. In future years the Commission intends
to tabulate the relative atomic masses within the isotopic composition tables. In this
year's Table of relative atomic masses of selected radionuclides (Table 5) the values are
again those recommended by A.H. Wapstra (Ref. 47) and the half—lives were provided by N.E.
Holden (Ref. 67). The latest atomic mass data were surveyed and no significant changes have
resulted.
OTHER PROJECTS
The Commission contemplates issuing a four or five place table of atomic weights in order to
provide practicing chemists with all the necessary data but no more, and to avoid at the
same time quoting uncertainties that do not affect everyday use of the data.
The four and five place values will change very infrequently compared to the definitive
table. In addition, the Commission will continue to publish the definitive Table of Standard Atomic Weights biennially, and plans to unify, as far as possible, the footnotes or
annotations in all tables to simplify their understanding.
TABLE 5. Relative Atomic Masses and Half—Lives of Selected Radionuclides
Atomic
Name
Mass
number
Relative
atomic mass
Symbol
number
Technetium
Tc
43
97
98
99
Promethium
Pm
61
145
144.913
147
146.915
96.906
97.907
98.906
Half—life
+
2.6x1O6
4.2x1O6
2.l3xlO5
a
a
a
18.
2.62
a
a
COMMISSION ON ATOMIC WEIGHTS AND ISOTOPIC ABUNDANCIES
2382
TABLE 5. Relative Atomic Masses and Half—Lives of Selected Radionuclides (Cont'd)
Name
Polonium
Astatine
Symbol
Atomic
number
Po
84
At
85
Mass
number
Relative
atomic mass
208
209
210
207.981
208.982
209.983
209
210
211
208.986
209.987
210.987
Half—life
2.90
102.
138.38
+
a
a
d
7.21
h
h
h
5.4
8.1
Radon
Rn
86
211
222
210.991
222.018
14.6
3.824
h
d
Francium
Fr
87
212
222
223
211.996
222.018
223.020
19.3
m
m
226
228
226.025
228.031
Radium
Ra
88
Actinium
Ac
89
225
227
225.023
227.028
Thorium
Th
90
230
232
230.033
232.038
230
233
230.035
231.036
233.040
233
234
235
236
238
Protactinium
Pa
91
231
Uranium
U
92
5.75
a
a
10.0
21.77
d
a
1600.
7.7x104
1.4Ox1O'°
a
a
d
a
d
233.040
234.041
235.044
236.046
238.051
l.59x105
2.44x105
7.04x108
2.34xl07
4.47x109
a
a
a
a
1.1x105
2.14x106
a
a
Np
93
236
237
236.047
237.048
Plutonium
Pu
94
238
242
244
238.050
239.052
240.054
241.057
242.059
244.064
241
m
3.28x104
27.0
Neptunium
239
240
15.
22.
17.4
87.7
2.41x104
6.54x103
14.7
3.8x105
8.3x107
a
a
a
a
a
a
a
Americium
Am
95
241
243
241.057
243.061
Curium
Cm
96
242
243
244
245
242.059
243.061
244.063
245.065
246
247
248
250
246.067
247.070
248.072
250.078
4.71x103
1.55x107
3.5x105
8.x103
a
a
a
a
247
247.070
249.075
1.4x103
3.2xl02
a
d
Curium
Berkelium
Bk
97
249
Californium
Cf
98
248
249
251
252
254
248.072
249.075
251.080
252.082
254.087
4.32x102
7.37x103
163.
28.5
18.1
8.5x103
334.
3.51x102
9.0x102
2.64
6.xlO
a
a
d
a
a
a
d
a
a
a
d
Atomic weights of the elements 1979
2383
TABLE 5. Relative Atomic Masses and Half—Lives of Selected Radionuclides (Cont'd)
Name
Symbo 1
Atomic
number
Einsteinium
Es
99
252
253
254
252.083
253.085
254.088
472.
20.47
276.
d
d
d
Fermium
Fm
100
255
257
255.090
257.095
20.1
100.5
h
d
Mass
numbe r
Relative
atom ic mas s
+
Hal f—l i fe
+ayear; dday; hhour; mminute.
REFERENCES
1. A.E. Cameron and E. Wichers, Report of the International Commission on Atomic Weights
1961, J. Amer. Chem. Soc. 84 4175 (1962).
2. G.P. Baxter and H.W. Starkweather, Proc. Nat. Acad. Sci. 14, 50 (1928).
3. G.P. Baxter, J. Amer. Chem. Soc. 50, 603 U28T
4.
5.
6.
7.
P. Eberhardt, 0. Eugster and K. Marti, Z. Naturforsch. 20a, 623 (1965).
J.R. Walton and A.E. Cameron, Z. Naturforsch. 21a, 115 TI66).
G.P. Baxter and H.W. Starkweather, Proc. Nat. Acad. Sci. 15, 441 (1929).
A.O.C. Nier,
Rev. 77 789 (195T
8. A.0.C. Nier, . Rev. 48, 283
(1935).
9. Atomic Weights of the Elements, 1969: Report of the IUPAC Commission on Atomic
Weights, Pure 2.L• Chem. 21, 95 (1970).
10. Atomic Weights of the Elements, 1971: Report of the IUPAC Commission on Atomic Weights,
Pure Appl. Chem. 30, 639 (1972).
11. G. Marinenko, Talanta 16, 1339 (1969).
12. R.G. Bates, E. Wickers, J. Res. Nat. Bur. Stand. (U.S.) 59, 9 (1957).
13. Atomic Weights of the Elements, 1975: Report of the IUPAC Commission on Atomic Weights,
Pure.
Chem. 47, 75(1976).
14. E.L. Garner, T.J. Murphy, J.W. Gramlich, P.J. Paulsen, I.L. Barnes,—J. Res. Nat.
Bur.
——
Stand.
15.
(u.s.) 79A, 713 (1975).
G.P.BaxterandA.Q. Butler, J. Amer. Chem. Soc. 48, 3117 (1926).
16. G.P. Baxter and A.Q. Butler, J. Amer.
Chem. Soc. 50,
17. A.0.C. Nier, !! Rev. 53, 282 Tii8).
408 (1928).
18.
R.F. Hibbs, U.S. A.E.C. Report, Y—646 (1950).
19. H.C. Mattraw and C.F. Pachucki, U.S.A.E.C. Report, AECU—1903 (1952).
20. J.E. Hogg, Can. J. Chem. 32, 1039 (1954).
21. V.H.W. Drawin, Nukleonik ! 109 (1958).
22. H.A. Belsheim, Iowa State Report, IS—T—217 (1968).
23. G.P. Baxter and L.W. Parsons, J. Amer. Chem. Soc. 43, 507 (1921).
24. G.P. Baxter and F.R. Hilton, Jr., J. Amer. Chem. Soc. 45, 694 (1923).
25.
G.P. Baxter and 5. Ishimura, J. Amer. Chem. Soc. 51 1729 (1929).
26. J.R. White and A.E. Cameron,
Rev. 74, 991 (1948).
27. Atomic Weights of the Elements, 1973: Report of the IUPAC Commission on Atomic Weights,
Pure
Chem. 37, 591, (1974).
28. I.L. Barnes, E.L. Garner, J.W. Gramlich, L.A. Machlan, J.R. Moody, L.J. Moore, T.J.
Murphy and W.R. Shields, Geochim. Cosmochim. Acta.,
4, 2 1197 (1973).
29. J.R. Sites, G. Consolazio and R. Baldock, Bull. Amer.
Soc. 28, 24 (1953).
30. N. Shima, C.E. Rees and H.G. Thode, Can. J.
56, 1333 (1978).
31.
G.P. Baxter, Mme. M. Curie, 0. Honigschmid, P. Le Beau and R.J. Meyer, J. Amer. Chem.
Soc. 54, 1269 (1932).
32. R.Whytlaw—Gray, H.5. Patterson and W. Cawood, Nature 127, 970 (1931).
33. F.W. Aston, Proc.
Soc. A126, 511 (1930).
34. A.0.C. Nier, . Rev. 79, 450 (1950).
35. R.E. Halsted,
Rev. 88, 666 (1952).
36. F. Everling, L.A. Konig, J.H.E. Mattauch and A.H. Wapstra, Nuci.
18, 529 (1960).
37. M.G. Inghram, R.J. Hayden and D.C. Hess, Jr., see G.T. Seaborg, I. Perlman, Rev. Mod.
20, 585 (1948).
38. B.J. Hogg and H.E. Duckworth, Can. J. . 32, 65 (1954).
39. 0. Honigschmid and Fr. Hirschbold—Wittner, Z. physik. Chem. 189A, 38 (1941).
40. G.W. Lugmair, N.B. Scheinin and K. Marti, Proc. Lunar, Sci. Conf., 6th, Geochim.
Cosmochim. Acta Suppl. 6, 2, 1419 (1975).
41. F.A. White, T.L. Collins, Jr. and F.M. Rourke,
Rev. 97, 566 (1955).
42. F.A. White, T.L. Collins, Jr. and F.M. Rourke,
. Rev. 101, 1786 (1956).
43. G.H. Palmer, J. Nucl. Energy 7, 1 (1958).
44. M.G. Inghram, R.J. Hayden and D.C. Hess, Jr.,
Rev. 73, 180 (1948).
45. W.T. Leland,
Rev. 76, 992 (1949).
.
—
—
CONMISSION ON ATOMIC WEIGHTS AND ISOTOPIC ABUNDANCIES
2384
46.
H.E. Duckworth, K.S. Woodcock and R.S. Preston, . Rev.
70, 479
(1950).
47. A.H. Wapstra and K. Bos, Atomic Data Nucl. Data Tables 19, 175 (1977).
48. 0. Hi5nigschmid, L. Birchenbach and E. Kothe, Sitzungsber
Akad. Wiss. 179
(1922).
49. 0. H6nigschmid and H. Striebel, Z. anor. allgem. Chem. 194, 293 (1930).
50. R.F. Hibbs, U.S.A.E.C. Report, AECU—556 (1949).
51. L.P. Dunston, J.W. Gramlich, I.L. Barnes and W.C. Purdy, J. Res Nat. Bur. Stand. (U.S.)
1
(1980).
52. 0. H6nigschmid and E. Wittner, Z. anor. allgem. Chem. 226, 289 (1936).
53. W.W. Boardman and A.B. Meservey, see R.E. Greene, C.A. Kienberger, and A.B. Meservey,
U.S.A.E.C. Report K—1201 (1955).
54. L.A. Smith, U.S.A.E.C. Report K—1462 (1961).
55. F.E. Senftle, L. Stieff, F. Cuttitta and P.K. Kuroda, Geochim. Cosmochim. Acta 11, 189
(1957).
56. G.A. Cowan and H.H. Adler, Geochim. Cosmochim. Acta 40, 1487 (1976).
57. R.F. Smith and J.M. Jackson, U.S.A.E.C. Report KY—581 (1969).
58. Atomic Weights of the Element, 1977: Report of the IUPAC Commission on Atomic Weights,
Pure 4.api Chem. 51, 405 (1979).
59. D. Ehhalt, G. Israel, W. Roether and W. Stich, J. Geophys. Res. 68, 3747 (1963).
60. V.A. Molochnova, M.M. Sokolov, A.V. Corey and N.M. Bugrov, Geokyimiya No. 5, 594
(1967).
61.
62.
63.
64.
H. Craig, Geochim. Cosmochim. Actal2, 133 (1957).
C. Lor4us and L. Merlivat, Proc. Grenoble Symposium IAHS Publ. No. 118, 127 (1977).
G.J. Wasserburg, T. Lee and D.A. Papanastassiou, Geophys. Res. Lett. 4 299 (1977).
M.T. McCulloch, J.R. DeLaeter and K.J.R. Rosman, Earth Planet Sci. Lett. 28, 308
(1976).
65. C.L. Smith, K.J.R. Rosman and J.R. DeLaeter, Int. J. Mass
Spectrom.
Ion
28, 7
(1978).
66. I.L. Barnes, E.L. Garner, J.W. Gramlich, L.A. Machlan, J.R. Moody, L.J. Moore, T.J.
Murphy and W.R. Shields, The Ioon 10, Number 3—4, 422 (Abstract, 5th Lunar Science
Conference, Houston, Texas, USA, March 18—22, 1974).
67. N.E. Holden, priv. comm., March 1980.