SPIRULINA-AN OVER VIEW
advertisement

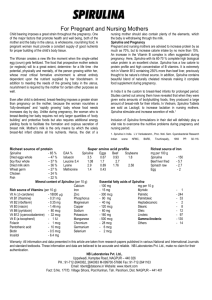
Academic Sciences International Journal of Pharmacy and Pharmaceutical Sciences ISSN- 0975-1491 Vol 4, Issue 3, 2012 Review Article SPIRULINA - AN OVERVIEW SHABANA KOUSER ALI*, ARABI MOHAMMED SALEH School of Bio Sciences and Technology, VIT University, Vellore. India. Email: shabanakouserali03@gmail.com Received: 28 Feb 2012, Revised and Accepted: 25 Mar 2012 ABSTRACT Arthrospira is a photosynthetic, filamentous, spiral-shaped, multicellular and blue- green micro alga. Cell division occurs by binary fission. As it contains chlorophyll a, like higher plants, botanists classify it as a micro alga belonging to Cyanophyceae class; but according to bacteriologists it is a bacterium due to its prokaryotic structure. Mexicans (Aztecs) started using this microorganism as human food. Its chemical composition includes proteins (55%-70%), carbohydrates (15%-25%), essential fatty acids (18%) vitamins, minerals and pigments like carotenes, chlorophyll a and phycocyanin. Pigments are used in food and cosmetic industries. Spirulina is considered as an excellent food, lacking toxicity and have anticancer, antiviral, immunological properties and it also acts as a potent antioxidant. There has been a significant change in Spirulina functions under stress conditions. Keywords: Arthrospira, Cyanophyceae. INTRODUCTION Systematics Spirulina named as Tecuitlatl by Aztecs, this means stone’s excrement during 16th century. Later, due to outbreak of contagious disease, new customs were adopted by people such as new foods, religious, political and social changes, and the topic of Tecuitlatl came to an end. It was not known till when man began to use microalgae, but at present this resources can be so called,” green tendency”20. Spirulina (Arthrospira) belongs to the oxygenic photosynthetic bacteria that cover the groups Cyanobacteria and Prochlorales11,49. Spirulina - “Small cakes made of a mud-like algae, which has a cheese-like flavor, and that natives took out of the lake to make bread,...". They are dried into cakes called "Dihe" or "Die’’. Back in 9th century, during Kanem Empire, only Spirulina had long history in Chad. In 1961, short story, The Voice of the Dolphins, Leo Szilard postulated the development of algae-based food supplement and named algae as “Amruss”. In early 1970s, First large scale production plant by Sosa Texcoco, was established13. With start of chemical analyses, the race to commercialization began13,34. Now, Spirulina is marketed and consumed in many different countries such as Germany, Brazil26,Chile, Spain, France, Canada, Belgium, Egypt, United States, Ireland, Argentina, Philippines, India, Africa, and other countries, where public administration, sanitary organisms and associations have approved human consumption20. Some of the best worldwide known Spirulina producing companies are: Earthrise Farms (USA), Cyanotech (USA), Hainan DIC Microalgae Co., Ltd (China), Marugappa Chettir Research Center (India), Genix (Cuba) and Solarium Biotechnology (Chile)1,7. A They are filamentous, non-heterocystous cyanobacteria that are generally found in tropical and subtropical regions in warm bodies of water with high carbonate/bicarbonate content, elevated pH, and salinity. Their large, gas-vacuolate filaments (3 to 12μm in diameter) are easily collected by filtration and other physical means of separation. Spirulina was isolated from fresh water sample in 1827 by Turpin13. Stizenberger’s studies on aseptate forms of Spirulina genus, and the septal forms of Arthrospira genus in 1892. Later, It is reunified the members of the two genera under the designation Spirulina17. In 1989, these microorganisms were classified into two genera, according to a suggestion by Gomont in 189211. This classification is currently accepted 42, 48. Stanier and Van Neil40 incorporated green-blue algae into the prokaryote kingdom and named these microorganisms as cyanobacteria and this was accepted and first published in 1974 in the Bergey`s Manual of Determinative Bacteriology19. Morphology Arthrospira maxima are filamentous cyanobacteria recognizable by the main morphological feature of the genus: the arrangement of the multicellular cylindrical trichomes in an open left-hand helix along the entire length. B Fig. 1: Light micrograph of Arthrospira maxima. B. Light micrograph of Arthrospira platensis. Bar represents 20μm. Ali et al. Under light microscopy, the blue-green non-heterocystous filaments, composed of vegetative cells that undergo binary fission in a single plane, show easily-visible transverse cross-walls. Filaments are solitary and free floating and display gliding motility. The trichomes, enveloped by a thin sheath, show more or less slightly pronounced constrictions at cross-walls and have apices either slightly or not at all attenuated. Apical cells may be broadly rounded or pointed and may be capitate and calyptrate. The width of the trichomes, composed of cylindrical shorter than broad cells varies from about 6 to 12μm (16μm) in a variety of forms. The helix pitch (h) is determined by the equation h=2Πrcotα Where, r is the radius of the cylinder surface to which the helix belongs and a is the angle formed by the helix and the cylinder generatrices and represents the slope of the helix curve. In many strains of these two species, the helix pitch varies from 12 to 72 μm. Also the helix diameter varies, ranging from about 30 to 70μm. Environmental factors, mainly temperature43, physical and chemical conditions, may affect the helix geometry. One drastic alteration of [ Int J Pharm Pharm Sci, Vol 4, Issue 3, 9-15 this geometry is the reversible transition from helix to spiral shape after transferring the filaments from liquid to solid media, first observed by46. Although the helical shape of the trichome is considered a stable and constant property maintained in culture, there may be considerable variation in the degree of helicity between different strains of the same species and within the same strain. Even in natural monospecific populations, variations in trichome geometry may be observed. Moreover, straight or nearly straight spontaneous culture variants have been repeatedly reported47. Once a strain has converted to the straight form, both naturally or after physical or chemical treatments, such as UV radiation or chemicals, it does not revert back to the helical form. This is due to a mutation affecting some trichomes during certain growth conditions. The common occurrence of straight trichomes, in the cultures of Arthrospira, may suggest that the helicity character is carried on plasmids. However, no plasmids have been observed in the strains checked. When, in a culture of a helically coiled strain, a few filaments happen to become straight, they tend to become predominant. This is probably due to competition between the two morphones, as observed in A. fusiformis. C D C D Fig. 2: Morphology of Spirulina. (A) Optical microscopy (X400) of axenic S. platensis. (Photo by G. Caretta) (B) Scanning electron micrograph of a trichome of axenic S. platensis. (Photo by R. Locci) (C) Electron micrograph of Arthrospira maxima in longitudinal section showing the division of the trichome into cells by cross-walls (s). Note the abundance of gas vacuoles (gv), the bundles of the thylakoid membranes (t), with associated phycobilisomes, many osmiophilic granules and ribosomes (r). (D) Electron micrograph of Arthrospira platensis in crosssection showing the multilayered cell wall (cw) and the subcellular organization of the cytoplasm. Note the accumulation of polyglucan granules (pg) close to the longitudinal cell wall (cw) and in the interthylakoidal space. In the central cytoplasmic region there are some carboxysomes (cs) (arrows) and two cylindrical bodies (cb). Bar represents 0.5 μm. (C and D were adapted from48). 10 Ali et al. Ultra-Structure Prokaryotic organization with fibrils of DNA region, photosynthetic system, pluri-stratified cell wall, capsule, ribosomes and numerous inclusions13. Cell wall is made of four numbered layers: L I, L II, L III and L IV (Ciferri 1983). The LI layer is not digestible by human beings as contains b-1, 2-glucan, the L II layer contains protein and lipopolysaccharides are favorite reasons for the easy human digestion of Spirulina5. Life Cycle There are three fundamental stages: Trichomes fragmentation, hormogonia cells enlargement and maturation processes, and trichome elongation. Then this mature trichomes get divided into filaments or hormogonia,cells in the hormogonias gets increased by binary fission,grows lengthwise and takes their helical form5. Fig. 3: Life cycle of Spirulina. Nutritional Composition Chemical analysis of Spirulina started in the year 1970 which showed Spirulina as an excellent source of proteins, vitamins and Int J Pharm Pharm Sci, Vol 4, Issue 3, 9-15 minerals41. Proteins constitute about 60%-70% of its dry weight13. Contains high provitamin A (β-carotene) 7, rich source of vitamin B12 and used in the treatment of pernicious anemia8,7. Lipids constitute about 4-7%29. It provides adequate amounts of iron in anemic pregnant women33. Carbohydrates are about 13.6%39. It has 2.2%-3.5% of RNA and 0.6 %-1% of DNA13. It also has few natural pigments such as carotenes, chlorophyll a and phycocyanin and these microorganisms are used as source of pigmentation for fish, eggs13, 20,37. It is reported the comparative β-carotene content in seven Spirulina strains among them Sp4 accumulated 231.7μg-1 dry weight36. Health Benefits Spirulina: has profound antioxidant potential, Its true healthprotective merit has only recently been discovered. phycocyanobilin (PCB), the chromophore bound to chief protein, phycocyanin, can function as a potent inhibitor of NADPH oxidase, the enzyme complex that is the chief source of pathological oxidant stress in a wide range of health disorders28. It appears to mimic the physiological activity of free bilirubin15. NADPH oxidase overactivity in disorders had suggested that ample intakes of Spirulina may prevent and has therapeutic potential with respect to many vascular diseases (including atherogenesis, hypertension, and congestive heart failure), cancers, complications of diabetes, and a range of neurodegenerative, fibrotic, or inflammatory disorder28. Oral administration of phycocyanin or of whole Spirulina has exerted central neuroprotective effects in rodent studies – an observation which strongly suggests that PCB can transit the blood– brain barrier16. Spirulina is an ideal food and dietary supplement for the 21st century by the Food and Agriculture Organization (FAO) of the United Nations32. The authentication of food ingredients is of crucial concern to food processors since the purity of food ingredients is easily subject to abuse by unscrupulous suppliers13, 34. Recent technological advances for the determination of food authenticity, Trends in Food Science and Technology7. Spirulina powder is one such food product that is easily subject to tampering. Fig. 4: Phycocyanobilin of Spirulina and Bilirubin of Higher fauna can mimic their activity Antiviral activity of Spirulina Spirulina has all bio-chemicals in its constitution that can build a healthy immune system, which scavenges free radicals as well. Compounds extracted from Arthrospira have inhibitory activity against a wide range of viruses such as HIV-1, HSV-1, HSV-2, HCMV, influenza type A, measles, etc. Extracts from cyanobacteria have antimutagenic and anticancer effect and can prevent development and growth of tumors as well as inhibit metastasis or proliferation of cancer cells14. Spirulina platensis was shown to minimize HIV-I replication in human T-cell lines, peripheral blood mononuclear cells (PBMC), and Langerhans cells (LC). Extract concentrations ranging between 0.3 11 Ali et al. and 1.2μg/ml reduced viral production by approximately 50% in PBMCs. A platensis extracts possess antiretroviral activity that may be of potential clinical interest2. Anti-Cancer Effects Several studies have shown that Spirulina or its extracts can prevent or inhibit cancers in humans and animals. In vitro studies suggest the unique polysaccharides of Spirulina enhance cell nucleus enzyme activity and DNA repair synthesis6. Immunological Applications NF-kappa B directed luciferase expression was enhanced by Immulina from Spirulina treatment when cells were co-transfected with vectors expressing proteins supporting TLR2 -(CD14 and TLR2) but not TLR4 -(CD14, TLR4, MD-2) dependent activation4. Spirulina or Arthrospira is a blue-green alga that became famous after it was successfully used by NASA as a dietary supplement for astronauts on space missionary, it inhibited the release of histamine by mast cells, and this alga may improve several symptoms of antiallergic effects25. Spirulina has no effect on chronic fatigue3. Spirulina extract (250mg) plus zinc (2mg) twice daily for 16 weeks may be useful for the treatment of chronic arsenic poisoning with melanosis and keratosis30. The first human feeding study that demonstrates the protective effects of Spirulina towards allergic rhinitis27. Spirulina helps to protect against certain nutritional deficiencies. It plays in the prevention of cancer, cellular ageing, infectious diseases and reduced immune system efficiency, as well as playing an important part in the functioning of the medulla (stimulation of the erythropoiesis)18. Aqueous extract of Spirulina, has a protective effect against apoptotic cell death due to free radicals12. Spirulina was a stronger inhibitor than Chlorella. Annexin-V staining showed that aqueous extract of Spirulina induced apoptosis of HSC after 12 h of treatment. In addition, the aqueous extract of Spirulina triggered a cell cycle arrest of HSC (hepatic stellate cell) at the G2/M phase50. Cultivation of Spirulina platens under salt stress conditions 0.02M (control), 0.04 and 0.08M NaCl led to a remarkable alteration of algal metabolism as well as an enhancement or induction of biologically active compounds. Concerning algal growth, salt stress caused a decrease in dry weight, chlorophyll a content as well as certain xanthophylls (neoxanthin and violaxanthin), while βcarotene production was stimulated especially at higher salt concentrations. Biochemical analysis of salt stressed algae revealed that lipid content was slightly increased together with certain saturated and unsaturated fatty acids especially the polyunsaturated ones (γinolenic acid, omega 3 fatty acid). Phosphate buffer and water extracts of the algae exhibited antiviral activities against both Hepatitis-A-virus-type-MBB (HAV-MBB strain, RNA virus) and Herpes simplex-virus-type-1 (HSV-1, DNA virus). Water extracts were found to be more effective than phosphate Int J Pharm Pharm Sci, Vol 4, Issue 3, 9-15 buffer extracts in inducing antiviral activities (98%) especially against HSV-1 virus. The same water extract of the salt stressed algae demonstrated higher anti-coagulating activity compared with those of heparin and the positive control measured by clotting time assay38. The salt-tolerant hypercarbonate strain CS-328 was grown in a medium containing 0.24 to 1.24M sodium, resulting in increased biosynthesis of soluble carbohydrates to up to 50% of the dry weight at 1.24M sodium. For cells grown in 1.24M NaCl, the fermentative yields of acetate, ethanol, and formate increase substantially to 1.56, 0.75, and 1.54mmol/(g [dry weight] of cells / day), respectively (36-, 121-, and 6-fold increases in rates relative to cells grown in 0.24M NaCl). Catabolism of endogenous carbohydrate increased by approximately 2-fold upon hypoionic stress. For cultures grown at all salt concentrations, hydrogen was produced, but its yield did not correlate with increased catabolism of soluble carbohydrates. Instead, ethanol excretion becomes a preferred route for fermentative NADH reoxidation, together with intracellular accumulation of reduced products of acetyl coenzyme A (acetyl-CoA) formation when cells are hypoionically stressed. In the absence of hypoionic stress, hydrogen production is a major beneficial pathway for NAD+ regeneration without wasting carbon intermediates such as ethanol derived from acetyl-CoA. This switch presumably improves the overall cellular economy by retaining carbon within the cell until aerobic conditions return and the acetyl unit can be used for biosynthesis or oxidized via respiration for a much greater energy return10. Salt stress leads to a modification of the QB niche at the acceptor side and an increase in the stability of the S2 state at the donor side, which is associated with a dissociation of the PsbO protein21. It resulted in a significant decrease in photosynthetic oxygen evolution activity and PSII electron transport activity, but a significant increase in PSI electron transport activity. In addition, it resulted in a decreased electron transport per PSII reaction center, but an increased absorption per PSII reaction center. (Tao Zhang et al., 2010). D1 protein turnover is involved in protection of Photosystem II against UV-B induced damage in the cyanobacterium Arthrospira22. Heat stress inhibited the maximum efficiency of PSII photochemistry significantly and showed no effects on the stability of the S2QA− and S2QB− states and that the different populations of the active PSII reaction centers show different sensitivity to heat stress in Spirullina platensis cells9. Malondialdehyde (MDA), superoxide dismutase (SOD) and proline contents increased under the heavy metal stress, corresponding to the concentration of the metal ion in the culture medium. Increased amount of MDA was indicative of formation of free radicals in the test microorganism under heavy metals stress, while increased levels of SOD and proline pointed to the occurrence of a scavenging mechanism in the cyanobacterium Spirulina platensis-S5 31. S. platensis might play a role in reducing the toxic effect of cadmium and its antioxidant properties seem to mediate such a protective effect 24. Table 1: Quantity of Spirulina proteins and other foods20 Food Type Spirulina powder Whole Dried egg Beer Yeast Skimmed powdered milk Whole soybean flour Parmesan Cheese Wheat germ Peanuts Chicken Fish Beef meat Crude Protein % 65 47 45 37 36 36 27 26 24 22 22 12 Ali et al. Int J Pharm Pharm Sci, Vol 4, Issue 3, 9-15 Table 2: Vitamins in Spirulina powder7 Vitamins Provitamin A (β-carotene) Vitamin E Thiamin B1 Riboflavin B2 Niacin B3 Vitamin B6 Vitamin B12 Folic acid Biotin Phantothenic acid Vitamin K mg 100 g-1 2.330.000 IU kg –1 140 100 a-tocopherol eq: 3.5 4 14 0.8 0.32 0.01 0.01 0.1 2.2 Table 3: Fatty acid composition of Spirulina platensis powder29 Fatty acid (C14) Myristic acid (C16) Palmitic acid (C16:1)D9 Palmitoleic acid (C18:1)D9 Oleic acid (C18:2)D9,12 Linoleic acid (C18:3)D9,12,15 g-Linolenic acid Others Fatty acids (%) 0.23 46.07 1.26 5.26 17.43 8.87 20.88 Table 4: Minerals in Spirulina powder7 Mineral Calcium Chromium Copper Iron Magnesium Manganese Phosphorus Potassium Sodium Zinc Table 5: Pigments in Spirulina powder7 Pigments Carotenoids Chlorophyll a Phycocyanin mg 100g-1 370 1000 14000 Table 6: Some commercial producers of Spirulinaª 48 b 32 Name of Company Earthrise Farms Location Calipatria, California, USA Myanma Microalgae Biotechnology Project Cyanotech Corporation Yangon, Myanmar Hainan DIC Microalgae Co., Ltd Ballarpur Industries Ltd Nao Pao Resins Chemical Co., Ltd Kailua Kona, Hawaii, USA China Nanjangud, Mysore District, India Tainan, Taiwan, ROC Neotech Food Co., Ltd Banpong, Rajburi, Thailand Siam Algae Co., Ltd. Solarium Biotechnology Thailand La Huayca, Chile Genix mg 100g-1 700 0.28 1.2 100 400 5 800 1400 900 3 Cuba Total area ª Intensive ponds, total area 150.000m2 ª Mainly native ponds with a total area 130.000m2 ª Intensive ponds, total area 100.000m2 b Total area 100.000m2 ª Intensive ponds, total area 54.000m2 ª Intensive ponds, total area 50.000m2 ª Intensive ponds, total area 50.000m2 b Intensive ponds, total area 45.000m2 b Total area 30.000m2 b Intensive ponds, total area 24.000m2 Production (ton) ª 1995: 360 ª 1996: 400 b 2002: 450 ª 1995: 32 ª 1996: 40 ª 1995: 250 ª 1996: 300 b 2002: 330 ª 1994 - 1995 : 25 ª 1995 - 1996: 85 ª 1995: 70 ª 1996: 80 ª 2000: 150 ª 1995: 30 ª 1996: 40 b 2001: 100 2002: 135 2000 (Oct-Dec): 4.5 b 2001: 28,6 b 2002 (Jan - Oct): 13 b b 13 Ali et al. Int J Pharm Pharm Sci, Vol 4, Issue 3, 9-15 CONCLUSION Spirulina is claimed as a non-toxic, nutritious food, with some corrective properties against viral attacks, anemia, and tumoral growth and as a source of the yellow coloration of egg yolk when consumed by hens, and growth. This contains proteins, carbohydrates, essential fatty acids, vitamins, minerals, carotenes, chlorophyll a and phycocyanin. There has been a significant change in functional properties of Spirulina under stressed conditions (salt and heat). Awareness of the better nutritional quality of sea food proteins and lipids will soon make them a major source of protein in the human diet. 14. 15. 16. REFERENCES 1. 2. 3. 4. 5. 6. 7. 8. 9. 10. 11. 12. 13. Ayala, F. Guía sobre el cultivo de Spirulina. In: Biotecnología de Microorganismos Fotoautótrofos. Motril, Granada, España.1998, p. 3-20. Ayehunie, S., Belay, A., Baba, T.W., Ruprecht, R.M. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology Volume 18, Issue 1, 1 May 1998, Pages 7-12 . Baicus, C., Baicus, A. Spirulina did not ameliorate idiopathic chronic fatigue in four N-of-1 randomized controlled trials. Phytotherapy Research Volume 21, Issue 6, June 2007, Pages 570-573 Balachandran, P., Pugh, N.D., Ma, G. and Pasco, D.S. Toll-like receptor 2-dependent activation of monocytes by Spirulina polysaccharide and its immune enhancing action in mice , International Immunopharmacology ,Volume 6, Issue 12, 5 December 2006, Pages 1808-1814. Balloni, W., Tomaselli, L., Giovanetti, L. and Margheri, M.C. 1980. Biologia fondamentale Del genere Spirulina. In: Cantarelli, C., Ciferri, O., Florenzano, G., Kapsiotis, G., Materassi, R., Treccani, U., Eds. Progetto finalizzato ¨Ricerca di nuove fonti proteiche e di nuove formulazioni alimentari¨. Atti del Convegno: Prospettive della coltura di Spirulina in Italia. Consiglio Nazionale delle Richerche. Firenze-Academia dei Georgofili, CNR, Tipografia Coppini; pp.49-82. 6.Baojiang G., et al. Study on Effect and Mechanism of Polysaccharides of Spirulina platens is on Body Immune Functions Improvement. Second Asia-Pacific Conference on Algal Biotechnology, April 25-27, 1994, p. 24. Belay, A. 1997. Mass culture of Spirulina outdoors – The Earthrise Farms experience. In: Vonshak, A., Ed. Spirulina platensis (Arthrospira): Physiology, cell-biology and biotechnology. Taylor and Francis. London. pp. 131-158. Becker, E.W. 1984. Nutritional properties of microalgal potentials and constraints. In: Richmond A, Ed. Handbook of microalgal mass culture. CRC Press, Inc, Boca Ratón; pp. 339408. Binbin Zhao, Jia Wang, Hongmei Gong, Xiaogang Wen, Haiyun Ren and Congming Lu. Effects of heat stress on PSII photochemistry in a cyanobacterium Spirulina platensis. Plant Science Volume 175, Issue 4, October 2008, Pages 556564. Carrieri, D., Momot, D., Brasg, I.A., Ananyev, G., Lenz, O., Bryant, D.A. Dismukes, G.C. Boosting autofermentation rates and product yields with sodium stress cycling: Application to production of renewable fuels by cyanobacteria. Applied and Environmental Microbiology, Volume 76, Issue 19, October 2010, Pages 6455-6462. Castenholz, R.W., and Waterbury, J.B. Oxygenic photosynthetic bacteria. Section 19, In: Staley, J.T., Bryant, M.P., Pfenning, N., Holt, J.G., Eds. Bergey’s Manual of Systematic Bacteriology. Vol. 3, Williams and Wilkins Co, Baltimore, USA.1989, pp 17101806. Chu, W.L., Lim, Y.W., Radhakrishnan, A.K., Lim, P.E. Protective effect of aqueous extract from Spirulina platens is against cell death induced by free radicals, BMC Complementary and Alternative Medicine Volume 10, 21 September 2010, Article number 53 . Ciferri, O. 1983. Spirulina, the edible microorganism. Microbiol. Rev. 47:551-578. Cordella, I. Moussa, A.C. Martel, N. Sbirrazzuoli and L. Lizzani-Cuvelier, Recent developments in food characterization and adulteration detection: Technique- 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. oriented perspectives, Journal of Agricultural and Food Chemistry 50 (7) (2002), pp. 1751–1764. Duda-Chodak, Aleksandra, Wajda, Lukasz, Kubica, Maria, Uniwersytet Rolniczy W Krakowie, Wplyw Bakterii Rodzaju Arthrospira Na Funkcjonowanie Ukladu Immunologicznego. Post. Mikrobiol. 2010. 49 (1):15-23. F. Jiang, S.J. Roberts, S. Datla and Dusting, G.J. NO modulates NADPH oxidase function via heme oxygenase-1 in human endothelial cells. Hypertension 48 (5) (2006), pp. 950–957. G. Chamorro, M. Perez-Albiter, N. Serrano-Garcia, J.J. MaresSamano and P. Rojas. Spirulina maxima pretreatment partially protects against 1-methyl-4-phenyl-1, 2, 3, 6tetrahydropyridine neurotoxicity, Nutr Neurosci 9 (5–6) (2006), pp. 207–212. Geitler, L. Cyanophyceae. In R. Kolkwitz(ed.), L. Rabenhorst's Kryptogamen-Flora von Deutschland, Osterreich und der Schweiz, vol. 14. Akademische Verlag, Leipzig.1932,p.916-931. Girardin-Andréani, C. Spirulina: Blood supply, immune system and cancer. Faculté Chirurgie Dentaire de Nancy. Faculté de Médecine de Lyon, Laboratoire Phytocorsa, Route de Pinarello, Sainte-Lucie-de-Porto-Vecchio, France. Phytotherapie ,Volume 3, Issue 4, August 2005, Pages 158-161 . Guglielmi, G., Rippka, R., and Tandeu de Marsac, N. Main properties that justify the different taxonomic position of Spirulina sp. and Arthrospira sp. among cyanobacteria. In: Doumenge, F., Durand-Chastel, H., Toulemont, A., Eds. Spiruline algue de vie. Bulletin de l´Institut Océanographique Monaco. Musée Océanographique. Numéro spécial,1993, 12:13-23. Henrikson, R. Microalga Spirulina, superalimento del futuro. Ronore Enterprises. 2ª ed. Ediciones Urano, Barcelona, España.1994, pp. 222. Hongmei Gong, Yunlai Tang, Jia Wang, Xiaogang Wen, Lixin Zhang and Congming Lu. Characterization of photosystem II in salt-stressed cyanobacterial Spirulina platens is cells. Biochimica et Biophysica acta 1777:2008, pp. 488-495. Hongyan Wu, Leyla Abasova, Otilia Chereg, Zsuzsanna Deák, Kunshan Gao and Imre Vass. D1 protein turnover is involved in protection of Photosystem II against UV-B induced damage in the cyanobacterium Arthrospira (Spirulina) platensis January 2011. Jourdan, Journal of Photochemistry and Photobiology B: Biology. Solarium Spirulina farm in the Atacama Desert (North Chile). In: Doumenge, F., Durand-Chastel, H., Toulemont, A., Eds. Spiruline algue de vie. Bulletin de l´Institut Océanographique Monaco. Musée Océanographique. Numéro spécial,1993, 2:191-194. Karadeniz, A., Cemek, M. and Simsek, N. The effects of Panax ginseng and Spirulina platens is on hepatotoxicity induced by cadmium in rats. Ecotoxicology and Environmental Safety January 2009, 72(1): pp. 231-235. Karkos, P.D. , Leong, S.C., Karkos, C.D., Sivaji, N. and Assimakopoulos, D.A. Spirulina in clinical practice: Evidencebased human applications, Evidence-based Complementary and Alternative Medicine. Volume 2011: Article number 531053. Lacaz, R., and Nascimento, E. Produçáo de biomassa de Spirulina máxima para alimentaçáo humana e animal. Rev Microbiol,1990,21:85-97. Mao, T.K., Van De Water, J., Gershwin, M.E. Effects of a Spirulina-based dietary supplement on cytokine production from allergic rhinitis patients. Division of Rheumatology, University of California at Davis, School of Medicine, Davis, CA, United States. Journal of Medicinal Food Volume 8, Issue 1, March 2005, Pages 27-30. 28.M.F. McCarty, “Iatrogenic Gilbert sydrome” – a strategy for reducing vascular and cancer risk by increasing plasma unconjugated bilirubin, Med Hypoth 69 (2007), pp. 974–994. 29.Misbahuddin, M. , Maidul Islam, A., Khandker, S., Ifthaker-AlMahmudc, Islam, N., Anjumanara.Efficacy of Spirulina extract plus zinc in patients of chronic arsenic poisoning: A randomized placebo-controlled study. Clinical Toxicology, Volume 44, Issue 2, 1 April 2006, Pages 135-141 . Meenakshi Choudhary, Umesh Kumar Jetley, Mohammed Abash Khana, Naina Zutshi and Tasneem Fatma. Effect of heavy metal 14 Ali et al. 31. 32. 33. 34. 35. 36. 37. 38. 39. stress on proline, malondialdehyde, and superoxide dismutase activity in the cyanobacterium Spirulina platensis-S5. Ecotoxicology and Environmental Safety, Volume 66, Issue 2, February 2007, Pages 204-209. Othes, S., and Pire, R. Fatty acid composition of Chlorella and Spirulina microalgae species. J. AOAC Int2001. 84: 1708-1714. Pelizer,L.H. Pelizer, E.D.G. Danesi, C.D. Rangel, C.E.N. Sassano, J.C.M. Carvalho, S. Sato and I.O. Moraes, Influence of inoculum age and concentration in Spirulina platens is cultivation, Journal of Food Engineering 56 (4), 2003,pp. 371–375. Puyfoulhoux, G., Rouanet, J.M,, Besancon, P., Baroux, B., Baccou, J.C., and Caporiccio, B.Iron availability from iron-fortified Spirulina by an in vitro digestion/Caco-2 cell culture model. J Agric Food Chem.2001, 49: 1625-29. Reid et al.,L.M. Reid, C.P. O’Donnell and G. Downey, Recent technological advances for the determination of food authenticity, Trends in Food Science and Technology 17 (7) (2006), pp. 344–353. Richmond, A. Mass culture of cyanobacteria. In: Mann, N., Carr, N., Eds. Photosynthetic prokaryotes. 2nd Ed. Plenum Press, New York and London. pp. 181-210. Romay et al. (1998) Antioxidant and anti-inflammatory properties of Cphycocyanin from blue-green algae. Inflamm Res 1992,47(1):36-41. Saleh, A.M, P.K. Singh and Dolly Wattal Dhar. Comparative βcaroten content of Spirulina strains at different days of incubation. The Andhra Agric.2008, J 55(1): 61-62 . Saxena, P.N., Ahmad, M.R., Shyan, R., and Amla, D.V. Cultivation of Spirulina in sewage for poultry feed. Experientia,1983, 39:1077-1083. Shalaby, E.A., Shanab, S.M.M. and Singh, V. Salt stress enhancement of antioxidant and antiviral efficiency of Spirulina platensis. Journal of Medicinal Plant Research Volume 4, Issue 24, 18 December 2010, Pages 2622-2632 . Shekharam, K., Ventakaraman, L., and Salimath, P.Carbohydrate composition and characterization of two unusual sugars from Int J Pharm Pharm Sci, Vol 4, Issue 3, 9-15 40. 41. 42. 43. 44. 45. 46. 47. 48. 49. 50. the blue-green algae Spirulina platens is. Phytochem,1987, 26:2267-2269. Stanier, R.Y., and Van Niel, Y. The concept of a bacterium. Arch. Mikrobiol. 42:17-35. Switzer, L. 1980. Spirulina, the whole food revolution. Proteus Corporation, USA; 1962,pp. 1-69. Switzer, L. Spirulina, the whole food revolution. Proteus Corporation, USA;1980, pp. 1-69. Tomaselli, L., Palandri, M. and Tredici, M.on the correct use of Spirulina designation. Algological Studies,1996, 83:539-548. Van Eykelenburg, C. The ultrastructure of Spirula platensis in relation to temperature and light intensity.Antonie van Leeuwenhoek J. Microbiol. Serol.1979,45:369-390. Van Eykelenburg, C.Rapid reversible macromorphological changes in Spirulina platensis. Naturwissenschaften.1980,67:200-201. Van Eykelenburg, C. Some theoretical considerations on the in vitro shape of the cross-walls in Spirulina spp. J. Theor. Biol.1980, 82:271-282. Van Eykelnburg, C., Fuchs, A. and Schmidt, G.H.Anatomie Van Leewenbokek,1989, 45, 369. Vonshak, A.Appendices. In: Vonshak, A., Ed. Spirulina platensis (Arthrospira): Physiology, Cell biology and Biotechnology. Taylor and Francis, London, Great Britain; 1997,pp. 213- 226. Vonshak, A. and Tomaselli, L.Arthrospira (Spirulina): Systematics and ecophysiology. In: Whitton, A., Potts, M., Eds. The Ecology of Cyanobacteria. Kluwer Academic Publishers. The Netherlands,2000,pp. 505-522. Whitton, B. Diversity, ecology and taxonomy of the cyanobacteria. In: Mann N, Carr N, Eds. Photosynthetic prokaryotes. Plenum Press,1992, pp. 1-37. Wu, L.C. , Ho, J.A.A., Shieh, M.C. and Lu, I.W. Department of Applied Chemistry, National Chi-Nan University, Puli, Nantou, Taiwan..Antioxidant and Antiproliferative activities of Spirulina and Chlorella water extracts. Journal of Agricultural and Food Chemistry Volume 53, Issue 10, 18 May 2005, Pages 42074212. 15