Pre-AP Chemistry: Naming & Formula Writing Worksheet
advertisement
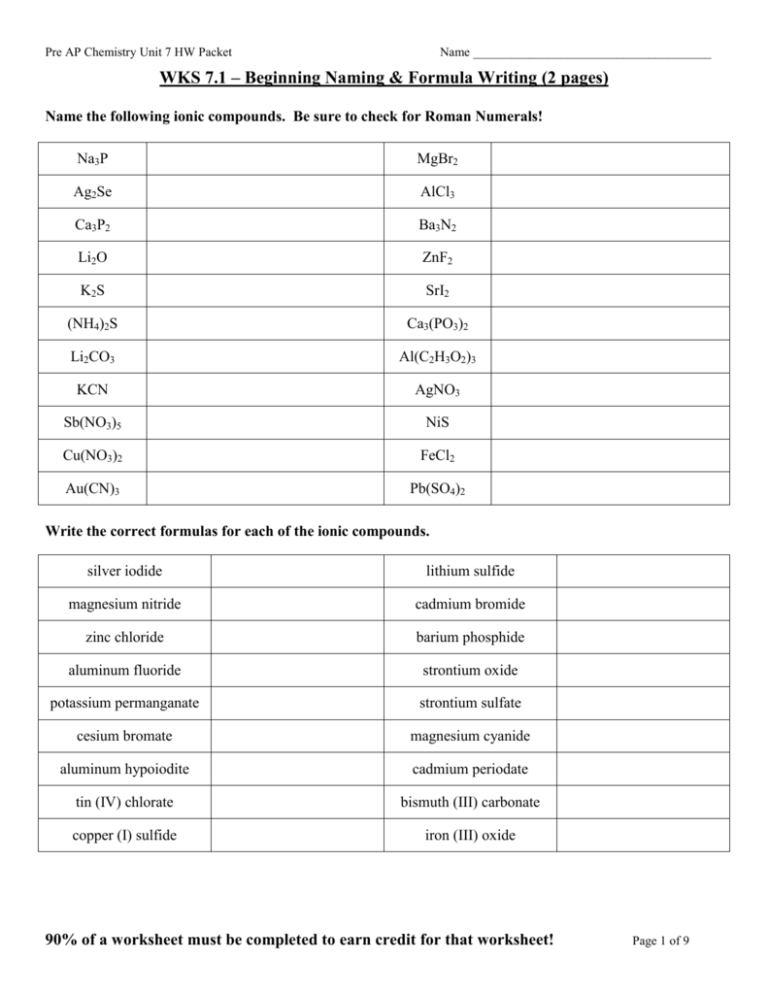
Pre AP Chemistry Unit 7 HW Packet Name ______________________________________ WKS 7.1 – Beginning Naming & Formula Writing (2 pages) Name the following ionic compounds. Be sure to check for Roman Numerals! Na3P MgBr2 Ag2Se AlCl3 Ca3P2 Ba3N2 Li2O ZnF2 K2 S SrI2 (NH4)2S Ca3(PO3)2 Li2CO3 Al(C2H3O2)3 KCN AgNO3 Sb(NO3)5 NiS Cu(NO3)2 FeCl2 Au(CN)3 Pb(SO4)2 Write the correct formulas for each of the ionic compounds. silver iodide lithium sulfide magnesium nitride cadmium bromide zinc chloride barium phosphide aluminum fluoride strontium oxide potassium permanganate strontium sulfate cesium bromate magnesium cyanide aluminum hypoiodite cadmium periodate tin (IV) chlorate bismuth (III) carbonate copper (I) sulfide iron (III) oxide 90% of a worksheet must be completed to earn credit for that worksheet! Page 1 of 9 Pre AP Chemistry Unit 7 HW Packet Name ______________________________________ WKS 7.1 – Beginning Naming & Formula Writing (continued) Write the correct formulas for each of the covalent compounds. nitrogen triiodide krypton hexafluoride iodine pentafluoride dinitrogen tetroxide diiodine pentoxide xenon trioxide disufur heptoxide sulfur trioxide arsenic pentafluoride dinitrogen pentoxide pentane cyclooctane cyclopropane heptane Name the following acids, or write the correct formula. H2SO4 (aq) HClO4 (aq) H2CO3 (aq) H3PO3 (aq) HMnO4 (aq) HI (aq) HNO2 (aq) HF (aq) sulfurous acid nitric acid hydrobromic acid phosphoric acid hypobromous acid hydrosulfuric acid 90% of a worksheet must be completed to earn credit for that worksheet! Page 2 of 9 Pre AP Chemistry Unit 7 HW Packet Name ______________________________________ WKS 7.2 – Formula Writing (1 page) 1. CLASSIFY each of the following substances as: Ionic Acid Inorganic Covalent Organic Covalent 2. Write a CORRECT chemical formula for each of the following substances. SUBSTANCE NAME Cadmium sulfide CLASSIFICATION FORMULA Lead (IV) permanganate Phosphoric Acid Sulfur trioxide Tetraphosphorus decoxide Sodium carbonate Cyclobutane Magnesium chloride hexahydrate Cesium bicarbonate Aluminum sulfide Decane Sulfur tetrafluoride Carbon disulfide Ammonium phosphite Dinitrogen tetrafluoride Dichlorine heptaoxide Hydrosulfuric acid Barium hyposulfite Diarsenic trioxide Calcium sulfate dihydrate Tin (II) nitride Zinc phosphate Sulfurous acid Perchloric acid 90% of a worksheet must be completed to earn credit for that worksheet! Page 3 of 9 Pre AP Chemistry Unit 7 HW Packet Name ______________________________________ WKS 7.3 – Formula Naming (1 page) 1. CLASSIFY each of the following substances as: Ionic Acid Inorganic Covalent Organic Covalent 2. Write a CORRECT chemical formula for each of the following substances. SUBSTANCE FORMULA SrS CLASSIFICATION NAME SeO2 H2SO3(aq) C7H16 Cu3P2 Cd(NO2)2 C3H8 ICl H2SO4 (aq) HCl (aq) MnO2 C9H18 NiCl2•6H2O Fe(C2H3O2)3 SnCl4•5H2O PH3 P2Se3 Hg(IO2)2 IF7 Cu3P Sn(CN)4 CoSO4•7H2O C2H6 HBrO4(aq) 90% of a worksheet must be completed to earn credit for that worksheet! Page 4 of 9 Pre AP Chemistry Unit 7 HW Packet Name ______________________________________ WKS 7.4 – Formula Naming, Round Two (1 page) 1. (NH4)2CO3 2. Na2CO3 3. Sn(HCO3)4 4. Hg(ClO)2 5. Pb(ClO2)2 6. HNO3 (aq) 7. HCl (aq) 8. H3PO4 (aq) 9. P2Br4 10. Fe(NO2)2 11. SbCl3 12. Ca(ClO4)2 13. HC2H3O2 (aq) 14. Al2(SO4)3 15. H2SO4 (aq) 16. Fe2O3 17. CrCl3 18. Cu2SO2 19. PF5 20. IF5 21. Ag2O 22. KClO4 23. Cl2O5 24. Pb3(PO4)4 25. CoF3 26. Ba(OH)2 27. B2Si 28. N2H4 29. P4S5 30. NaHCO3 31. Sr3(PO4)2 32. As4O10 33. ClF3 34. N2S 35. HgF2 36. LiMnO4 37. Si2Br6 38. Bi2(SO3)5 39. Pb(ClO3)2 • 3 H2O 40. SeF6 41. SBr2 42. Cl2O 43. P2O5 44. MgSO4 • 9 H2O 90% of a worksheet must be completed to earn credit for that worksheet! Page 5 of 9 Pre AP Chemistry Unit 7 HW Packet Name ______________________________________ WKS 7.5 – Formula Writing, Round Two (1 page) 1. Selenium trioxide 2. Iron (II) hypobromite 3. Copper (I) sulfate 4. Hexabromide monosilicide 5. Nitric Acid 6. Boron trihydride 7. Iodine pentabromide 8. Dichlorine heptaoxide 9. Nickel (III) iodide 10. Antimony (III) chloride 11. Ammonium phosphate 12. Hypoiodous Acid 13. Silicon dioxide 14. Carbon tetrachloride 15. Nickel (III) nitrate 16. Zinc bicarbonate 17. Cyclohexane 18. Dinitrogen monoxide 19. Dinitrogen tetroxide 20. Copper (II) sulfate pentahydrate 21. Pentane 22. Chromium (III) bisulfate 23. Carbonic Acid 24. Hydrophosphoric Acid 25. Perchloric Acid 26. Silver cyanide 27. Magnesium hydroxide 28. Ammonium Sulfide 29. Ammonium chlorate 30. Potassium oxide 31. Iron (III) sulfide 32. Diphosphorous pentoxide 33. Octane 34. Hydrobromic Acid 35. Aluminum sulfite 36. Cyclononane 37. Magnesium sulfate trihydrate 38. Bismuth (III) bicarbonate 39. Copper (II) carbonate 40. Fluorine tribromide 41. Iodous Acid 42. Hyposulfurous acid 90% of a worksheet must be completed to earn credit for that worksheet! Page 6 of 9 Pre AP Chemistry Unit 7 HW Packet Name ______________________________________ WKS 7.6 – Correcting Formulas (1 page) Each of these formulas or names have something wrong with them – charges not balanced, parentheses not used correctly, endings not changed, two cations or two anions together, elements in wrong order, and more. State what is incorrect about each of the following. 1. NaPO4 ___________________________________________________________________________ 2. MgOH2 __________________________________________________________________________ 3. HSO4 (aq) ________________________________________________________________________ 4. NH4K ___________________________________________________________________________ 5. Cl2Mg ___________________________________________________________________________ 6. Cu2S_____________________________________________________________________________ 7. C2H3O2H (aq) ______________________________________________________________________ 8. NH4Cl4 ___________________________________________________________________________ 9. MgClO2 ___________________________________________________________________________ 10. MgIO42 ____________________________________________________________________________ 11. LiS _______________________________________________________________________________ 12. Calcium chlorine _____________________________________________________________________ 13. Zinc (II) bromide _____________________________________________________________________ 14. Copper carbonate _____________________________________________________________________ 15. Sulfur cyanide _______________________________________________________________________ 16. Sulfic Acid __________________________________________________________________________ 17. Magnesium calcium ___________________________________________________________________ 18. Iron (3) chloride ______________________________________________________________________ 19. Tin (III) nitrate _______________________________________________________________________ 20. Dinitrogen chloride ___________________________________________________________________ 90% of a worksheet must be completed to earn credit for that worksheet! Page 7 of 9 Pre AP Chemistry Unit 7 HW Packet Name ______________________________________ WKS 7.7 – Writing Formulas, Counting Atoms (1 page) Write the formula for each of the following, and then answer the calculation for each. QUESTION CHEMICAL FORMULA ANSWER 1. How many total atoms are there in Calcium hydroxide? 2. How many total atoms are there in Tin (IV) carbonate? 3. How many atoms of oxygen are there in Magnesium bicarbonate? 4. How many total atoms are there in Acetic acid? 5. How many atoms of nitrogen are there in Ammonium nitride? 6. How many total atoms are there in Aluminum permanganate? 7. How many atoms of oxygen are there in Iron (III) perbromate? 8. How many atoms of sulfur are there in Lead (II) sulfate? 9. How many total atoms are there in Phosphoric acid? 10. How many total atoms are there in Diphosphorus trioxide? 11. How many atoms of hydrogen are there in Ammonium acetate? 12. How many atoms of cesium are there in Cesium phosphate? 13. How many total atoms are there in Copper (II) sulfite? 14. How many atoms of tin are there in Tin (IV) phosphate? 15. How many atoms of carbon are there in Barium Carbonate? 16. How many atoms of oxygen are there in Aluminum sulfite? 17. How many atoms of oxygen are there in Barium chlorate hexahydrate? 18. How many total atoms are there in Dinitrogen pentoxide? 19. How many total atoms are there in Copper (II) sulfate pentahydrate? 90% of a worksheet must be completed to earn credit for that worksheet! Page 8 of 9 Pre AP Chemistry Unit 7 HW Packet Name ______________________________________ WKS 7.8 – Balancing Reactions (1 page) Balance the following reactions, putting coefficient numbers in the spaces provided. If no coefficient is needed, simply leave the space blank – there is no need to place a “1” as a coefficient. 1. ____H3PO4 + ____HCl ____PCl5 + ____H2O 2. ____CaC2 + ____H2O ____C2H2 + ____Ca(OH)2 3. ____Fe(OH)3 ____Fe2O3 4. ____Ca(ClO3)2 ____CaCl2 + ____O2 5. ____Pb(NO3)2 ____PbO + ____NO2 + ____O2 6. ____C2H5OH + ____O2 ____CO + ____H2O + ____H2O 7. ____BaO + ____H2O ____Ba(OH)2 8. ____Ca + ____AlCl3 ____CaCl2 + ____Al 9. ____NH4NO3 ____N2O + ____H2O 10. ____Au2O3 ____Au + ____O2 11. ____H3PO3 ____H3PO4 + ____PH3 12. ____C4H10 + ____O2 ____CO2 + ____H2O 13. ____Fe2O3 + ____C ____CO + ____Fe 14. ____Fe3O4 + ____H2 ____Fe + ____H2O 15. ____I2 + ____HNO3 ____HIO3 + ____NO2 + ____H2 16. ____C6H6 + ____O2 ____CO2 + ____H2O 17. ____C2H5OH + ____O2 ____CO2 + ____H2O 90% of a worksheet must be completed to earn credit for that worksheet! Page 9 of 9




