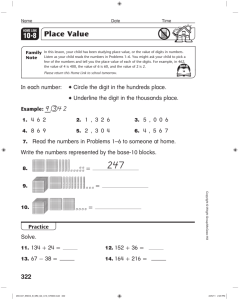
1 AP Chem Summer Assignment: Independent Study Chapters 1

AP Chem Summer Assignment:
Independent Study Chapters 1 and 2 of textbook
Read Chapters 1 and 2 of text book, these are a review of Chem 1
We will not be discussing these chapters in class.
Answer the questions in this packet to accompany Chapters 1 and 2
The answers to the questions in the packet will be posted on my website during the last week of August. You can check your answers then
Do the following questions from your text: These will be turned in and graded on Friday, September 7 th
.
Pages 31-35 : # 5,15,16,24,25,28,29,35,39,41,45,57,60,65,70
Pages 71-76 : # 1,9,27,29,31,33,39,41,59,61,63,64,65,68,69,71,73,75,
84,94,96
There will be a test on Chapters 1 and 2 on Friday, September 7.
1
AP Chemistry Unit I Chemical Foundations
Chapters 1 Matter and Measurement , Chapter 2-Atoms Molecules and Ions
Objective s
Chapter 1 Matter and Measurement
Recall a definition of chemistry
Understand the stages and process of scientific problem solving
List the states of matter, defining and listing characteristics of the common states
Compare and contrast physical and chemical changes
Differentiate among elements, mixtures and compounds
Differentiate between homogeneous and heterogeneous mixtures
Understand and use scientific notation
Define and convert among SI units and prefixes
Understand the concept of derived units and use relationships relating to density
Define uncertainty in a measurement and recall the rules for significant digits in calculations
Compare and contrast accuracy and precision
Interconvert between Celsius, Kelvin and Fahrenheit temperature scales
To state the fundamental chemical Laws of Conservation of Mass, Definite Proportions, and Multiple Proportions
Chapter 2 Atoms, Molecules and Ions
To Summarize Dalton’s Atomic Theory
To explain how the Experiments of Thomson, Millikan, and Rutherford led to the
Modern view of Atomic Structure
Define isotope, and describe how mass spectrometers determine the mass of an isotope
Calculate the Average atomic mass
Distinguish among terms chemical formula, empirical formula, molecular formula and structural formula
Determine the oxidation numbers of monatomic ions
State the formula and oxidation numbers of a variety of polyatomic ions
Distinguish among three types of inorganic substances: ionic compounds, acids, and binary molecular compounds
Name and write formulas for some simple Organic compounds
2
Classifying
Matter
http://wps.prenhall.com/wps/media/objects/165/169061/GIFS/AAAUASO0.JPG
3
Classify the following as element, compound, homogeneous mixture , heterogeneous mixture:
1.
Aqueous solution of sodium chloride ________________________
2.
Air_____________________________________________________
3.
Granite_________________________________________________
4.
Sodium chloride _________________________________________
5.
White Gold (gold and palladium in differing amounts) ____________
States of matter
Properties described on the macroscopic level:
•gas (vapor)_________________________________________.
•liquid : _______________________________________________
•solid : ________________________________________________
Properties described on the molecular level:
•gas : ___________________________________________________.
•liquid : ___________________________________________________
•solid : ___________________________________________________
Physical vs. Chemical Properties
A physical property is observed with the senses and can be determined without destroying the object. For example, color, shape, mass, length, and odor are all examples of physical properties
A chemical property indicates how a substance reacts with something else. The original substance is fundamentally changed in observing a chemical property. For example, the ability of iron to rust is a chemical property. The iron has reacted with oxygen, and the original iron metal is changed. It now exists as iron, a completely different substance.
Intensive properties do not depend on the amount of substance (for example, alcohol is flammable).
Extensive properties do depend on the amount (for example, the mass of the alcohol).
Classify the following properties as either chemical or physical by putting a check in the appropriate column. Also indicate if it is an intensive or extensive property.
Property
1.
weight
2.
blue color
3.
density
4.
flammability
5.
volume
4
Physical vs Chemical Changes
In a physical change , the original substance still exists, it has only changed in form. In a chemical change , a new substance is produced. Energy changes always accompany chemical changes.
Classify the following as being a physical or chemical change by writing a C or a P .
1.
When placed in water, a sodium pellet catches on fire as hydrogen gas is liberated and sodium hydroxide forms Sodium hydroxide dissolves in water.
2.
Sugar dissolves in water
3.
Hydrochloric acid reacts with potassium hydroxide to produce salt, water, and hat.
4.
Leaves change color.
5.
Potassium chlorate decomposes into potassium chloride and oxygen gas.
6.
Iron rusts.
7.
Distillation of a liquid
8.
Ice melting.
9.
Milk sours.
10.
evaporation
Counting Significant Digits
Quantities in chemistry are of two types:
Exact numbers – These result from counting objects such as desks (there are 24 desks in this room), occur as defined values (there are 100 cm in 1 m), or as numbers in formulas (area of a right triangle = ½ B x H). They (24, 100, 1, and ½ for these examples) all have an infinite(∞) number of significant digits. B and H are measurements and do not have an infinite number of digits.
Inexact numbers – These are obtained from measurements and require judgment.
Uncertainties exist in their values.
When making any measurement, always estimate one place past what is actually known.
For example, if a meter stick has calibrations to the 0.1 cm, the measurement must be estimated to the 0.01 cm. When making a measurement with a digital readout, simply write down the measurement. The last digit is the estimated digit.
5
Significant digits are all digits in a number which are known with certainty plus one uncertain digit. The following rules can be used when determining the number of significant digits in a number:
Rule
1. All nonzero numbers are significant.
2. All zeros between nonzero numbers are significant.
Example
132.54 g
Sig Digs
5
130.0054 m 7
3. Zeros to the right of a nonzero digit but to the left of an understood decimal point are not significant unless shown by placing a decimal point at the end of the number.
4. All zeros to the right of a decimal point but to the left of a nonzero digit are NOT significant.
5. All zeros to the right of a decimal point and to the right of a nonzero digit are significant.
A good way to remember which side to start on is:
190 000 mL 2
190 000. mL 6
0.000 572 mg 3
460.000 dm 6
decimal point present, start at the Pacific decimal point absent, start at the Atlantic
How many significant digits do each of the following numbers have?
1. 2.3000 x 10
6
_____________________ 6. 500.
2. 45.1 _____________________ 7. 970
_____________________
_____________________
3. 800000.103 _________________ 8. 0.002 _____________________
4 0.000000001500_________________ 9. 0.007 80 _____________________
5. 600 _____________________ 10. 145.55 _____________________
6
Rounding Rules
Calculators often give answers with too many significant digits. It is often necessary to round off the answers to the correct number of significant digits. The last significant digit that you want to retain should be rounded up if the digit immediately to the right of it is
(Each of the examples are being rounded to four sig digs):
Rule
….. greater than 5
….. 5, followed by a nonzero digit
….. 5, not followed by a nonzero, but has an odd digit directly in front of it.
Example
532.79
17.255 1
3 213.5
4 sig digs
532.8
17.26
3214
The last significant digit that you want to retain should stay the same if the digit immediately to the right of it is:
Rule
….. less than 5
….. 5, not followed by a nonzero digit, but has an even digit directly in front of it.
Example
5 454.33
0.007 85
4 sig digs
5 454
0.007 8
Round the following numbers to 3 sig digs.
1. 279.3 ___________ 3. 32.395 ___________ 5. 18.29
2. 42.353 ___________ 4. 32.25 ___________ 86 5 001
Applying significant digits to arithmetic operations
___________
___________
Addition and Subtraction – Look at the numbers being added or subtracted and identify which one has the lowest number of decimal places . Calculate the answer. Round the answer to the lowest number of decimal places .
14.565 + 7.32 = 21.885
7.32 has only 2 decimal places, so the answer should be rounded to 21.88
143.52 – 100.6 = 42.92
100.6 has only 1 decimal place, so the answer should be rounded to 42.9
Multiplication and Division – Look at the numbers being multiplied or divided and identify which one has the lowest number of significant digits . Calculate the answer. Round the answer to the lowest number of significant digits .
172.6 x 24.1 = 4159.66
24.1 has only 3 significant digits, so the answer should be rounded to 4160
172.6 ÷ 24.1 = 7.161 82
234.1 only has 3 significant digits, so the answer should be rounded to 7.16
Express each answer with the correct number of significant digits.
1.
320 x 24.9
0.080
2.
432.7 x 6.5 x .002300
62 x 0.103
3.
32.44 + 4.9- 0.304
82.94
4.
8.002 + 0 .3040
13.4 - 0.066 + 1.02
7
Temperature is defined as the average kinetic energy of the particles in a sample of matter. The units for this are o
C and Kelvin (K). Note that there is no degree symbol for
Kelvin. Absolute zero is ) Kelvin. This is the coldest possible temperature where all molecular motion ceases
Heat is a measurement of the total kinetic energy of the particles in a sample of matter.
The units for this are the calorie (cal) and the Joule (J).
The following equation can be used to convert temperatures from Celsius ( t ) to Kelvin
( T ) scales and fahrenheit to celsius and celsius to fahrenheit
T (K) = t ( o
C) + 273.15 t ( o
C) = T (K) - 273.15
You are simply subtracting 273.15 from your Kelvin temperature.
Convert the following:
1. 335C = _______________K
2 50.0 K = _______________C
8
SI Fundamental Units
http://wpscms.pearsoncmg.com/wps/media/objects/3661/3749680/Aus_content_01/Table01-03.jpg
SI Derived Units
- calculated from fundamental units http://cornellchem.wikispaces.com/Unit+2+-+Measurements+and+Calculations
SI Prefixes
http://wpscms.pearsoncmg.com/wps/media/objects/3661/3749680/Aus_content_01/Table01-03.jpg
9
Using Dimensional Analysis to convert among metric units
1.
Convert 1.09 ng to g
2.
Convert 90.1 ms to s
3.
Convert 145 pm to m
4.
Convert 1.55 kg/m
3
to g/L
5.
Determine the number of L in a box that measures 4cm x 8cm x 10cm.
(1 ml= 1 cm
3
)
6.
If a car has an EPA mileage rating of 30 miles per gallon, what is this rating in km/L?
7.
10
Accuracy versus Precision
Percent Error – Measures accuracy of measurement.
Calculate the percent error for the following problems. Show your work and use the correct number of significant digits in your answer.
1.
A student measures a rectangular piece of metal. According to the student, it has a length of 1.98 cm, a width of 5.15 cm, and a height of 1.03 cm and a mass of
77.89 g. The metal is iron, which has a density of 7.80 g/cm
3
. What is the percent error?
2.
A technician is making a 0.500 M salt solution for an experiment. The experiment will work as long as the solution is within 2.00 % of the accepted
0.500 M. In actuality, the solution was 0.522 M. Will the experiment work?
11
Density
The formula for density is d=m/v
The density of wáter at 22 C is 1 g/ml
1 cm
3
= 1 ml
1.
An irregularly shaped piece of metal with a mass of 125g is placed into a graduated cylinder that contains 25.00mL of water. This raises the water level to
56.00mL. What is the density of the metal?
2.
Calculate the mass of a block of iron (density = 7.86g/ml) with dimensions of
528mm x 67.4mm x 37.3mm.
3.
The volume of a red blood cell is about 9.00x10
-11 cm
3
. Assuming that red blood cells are spherical, what is the diameter of a red blood cell in inches?
4.
A fertilizer contains 21% nitrogen by mass. What mass of this fertilizer, in kilograms, is required for an application of 775g of nitrogen?
5.
A column of mercury is contained in a cylindrical tube. The tube has a diameter of 8.0 mm, and the height of the mercury column is 1.20 m. Given that the density of mercury is 13.6 g/mL and that the volume of mercury in the tube can be calculated by the relation V = πr
2 h (r = the radius of the tube and h = the height of the mercury column), calculate the mass of mercury present in the cylindrical tube.
12
Fundamental Chemical Laws and Dalton’s Atomic Theory
What Laws do Dalton’s atomic theory explain?
__________________________________________________________________
__________________________________________________________________
__________________________________________________________________
__________________________________________________________________
13
Thomson’s Cathode Ray Tube
Experiments
Describe Thomson’s Cathode ray tube experiment and describe the major conclusions of this experiment:
__________________________________________________________________
__________________________________________________________________
__________________________________________________________________
__________________________________________________________________
Describe this picture in terms of Thomson’s model
14
Millikan’s Oil Drop Experiment
What was Milikan able to determine from his oil drop experiment?
__________________________________________________________________
__________________________________________________________________
__________________________________________________________________
__________________________________________________________________
Radioactivity
Explain the behavior of alpha, beta and gamma rays as illustrated in the diagram above
__________________________________________________________________
__________________________________________________________________
__________________________________________________________________
__________________________________________________________________
15
Rutherford’s Gold Foil Experiment
Describe Rutherford’s Gold Foil Experiment and describe the major conclusions of this experiment:
__________________________________________________________________
__________________________________________________________________
__________________________________________________________________
__________________________________________________________________
Atomic theory Summary
Scientist
Thomson
Millikan
Experiment Knowledge gained Relating to
Rutherford
Chadwick
16
Modern view of Atomic Structure
Atomic number & the number of electrons, protons, and neutrons.
A
Z
X
is the general symbol for an isotope where A is the mass number (number of protons + number of neutrons), X is the element’s symbol, and Z is the atomic number (number of protons). This information can also be found on the periodic table. Use this information to fill in the chart for the following elements
Symbol
Atomic
Number
Atomic
Mass
Protons Electrons Neutrons Element Name
Gold copper nickel lead zinc
17
Isotopes and Mass Spectroscopy
18
Average Atomic Mass
Directions :
1.
Write the isotopes.
2.
Write the masses.
3.
Multiply the masses by the decimal equivalent of the relative abundance.
4.
Add the products. This is the average atomic mass.
Example:
A sample of cesium is 20.0 %
132
Cs ,75.00 %
133
Cs, and 5.0 %
134
Cs
132
Cs = 132 x 0.200 = 26.4
133
Cs = 133 x 0.7500 = 99.75
134
Cs = 134 x 0.050 = _6.7_
132.8
Determine the average atomic mass of the following mixtures of isotopes:
1. 80.0 % I-127, 17.0 % I-126, and 3.00 % I-128.
2. 50.0 % Au-197, 50.0 % Au-198.
3.
Naturally occurring chlorine molecules (chlorine is diatomic) have masses of
70, 72 and 74. They occur in the percentages of 56.25%, 37.50% and
6.250%. What is the average atomic mass of chlorine atoms?
19
Chemical Nomenclature Flowchart
Case 1: Ionic compounds containing monatomic ions (i.e. ions that can only have one charge)
Name of Compound = name of metal + name of non-metal w/ide suffix or name of polyatomic ion. No prefixes are used ! e.g. NaF = sodium fluoride; Na
3
PO
4
= sodium phosphate; (NH
4
)
3
PO
4
= ammonium phosphate
Case 2: Ionic compounds containing a metal that can form more than one ion
Name of Compound = name of metal, followed by charge of metal in Roman numerals in parentheses , followed by name of non-metal w/ -ide suffix or name of polyatomic ion. No prefixes are used ! e.g. PbCl
2
= Lead (II) chloride; Cu(NO
3
)
2
= copper (II) nitrate
Case 3 : Binary molecular compounds :
Name of Compound = name of first element + name of second element with -ide suffix.
Use prefixes (mono-, di-, tri-, tetra-, penta-, hexa-, hepta-, nona-, deca-) to indicate the number of atoms.
The mono prefix is not used with the first element. e.g. CO = carbon monoxide; NO
2
= nitrogen dioxide; N
2
O = dinitrogen monoxide; P
2
O
5
= diphosphorus pentoxide
Case 4: Binary acid solutions (i.e. binary acids dissolved in water = binary acids in aqueous solution)
Name of Compound = hydro + name of halogen w/ -ic suffix e.g. HF
(aq)
= hydrofluoric acid; HCl
(aq)
= hydrochloric acid
Unless stated otherwise assume the formula of a binary acid is for the acid dissolved in water. E.g. assume HCl = HCl
(aq)
Naming Oxoacids (i.e. compound with the general formula H x
MO y
, where M = nonmetal)
The name of an oxoacid is based on the name of the polyatomic ion from which the acid is derived.
Case 5: -ate
-ic
If the name of the polyatomic ion ends in
“-ate ,” the name of the corresponding acid ends in “-ic
acid.”
Polyatomic ion (-ate)
Acid (-ic) sulfate = SO
4
2-
H
2
SO
4
= sulfuric acid
Chlorate = ClO
3
1-
HClO
3
= chloric acid
Case 6: -ite
-ous
If the name of the polyatomic ion ends in
“-ite ,” the name of the corresponding acid ends in “-ous
acid.”
Polyatomic ion (-ite)
Acid (-ous) sulfite = SO
3
2-
H
2
SO
3
= sulfurous acid
Chlorite = ClO
2
1-
HClO
2
= chlorous acid
20
Write the formula.
1.
Ammonium Nitrate
2.
Calcium Bicarbonate or Calcium
Hydrogen Carbonate
3.
Barium Chlorate
4.
Dimercury (I) Iodide
5.
Hydronitric acid
6.
Plumbic Oxide or Lead (IV) Oxide
7.
Potassium Thiocyanide
8.
Perchloric acid
9.
Sulfur hexafluoride
10.
Zinc Hydroxide
11.
Potassium Sulfite
12.
Copper (I) Sulfide
13.
Potassium bisulfate
14.
Zinc Bromide
15.
Ferric Chromate
16.
Sodium Perchlorate
17.
Potassium Hypochlorite
18.
Magnesium Nitride
19.
Sodium Permanganate
20.
Potassium Permanganate
Write the name.
21.
Hg
2
(CN)
2
22.
H
2
SO
4
23.
Fe(C
2
H
3
O
2
)
2
24.
KClO
3
25.
PbF
2
26.
HBr
27.
N
2
O
4
28.
HgCrO
4
29.
Ag
3
PO
4
30.
K
2
Cr
2
O
7
31.
Ba
3
N
2
32.
NF
3
33.
CuBr
2
34.
(NH
4
)
2
S
35.
Ca(NO
3
)
2
36.
Zn(OH)
2
37.
NaHCO
3
38.
PbO
2
39.
KClO
4
40.
Hg
2
I
2
21
Mixed Practice
Write the correct formula, Write I, A, or M to the left to state what type of compound it is
1.
______calcium fluoride _________________________
2.
_______ferrous carbonate _________________________
3.
_______aluminum hydroxide __________________________
4. _______dinitrogen pentaoxide ___________________________
5. _______chromic acid ___________________________
6. _______nitrogen monoxide ___________________________
7. _______plumbic sulfate ___________________________
8. _______hydrophosphoric acid _____________________________
9.________tetraphosphorus decoxide ____________________________
Write the correct name. IF THERE ARE TWO WAYS TO NAME A COMPOUND
USE BOTH!!
9. ______ LiS ___________________________________________
10. ______NH
4
Cl _______________________________________
11. _______SnSO
4
____________________________________________
12. _______ H
3
PO
3
_____________________________________________
13. ________ P
2
O
5
____________________________________________
14. ________ CO ______________________________________________
15. _______H
3
N ______________________________________________
16. _______ CuS ________________________________________________
22
Naming Acids
“ate” becomes “____ic acid” I ate something ic ky
“ite” becomes “____ous acid”
“ide” becomes hydro ___ic acid
**in sulfur acids add “ur” in phosphorous acids add “or”
Write formula and oxidation number of anion, write correct formula for acid, name the acid: anion Anion formula
/ox #
Acid formula
Acid name bromate periodate carbonate sulfide chloride chloric thiosulfate dichromate chromate permanganate bromate iodite iodide perchloride phosphate hyporchlorite phosphide
23
Simple Organic Compounds
Alkanes
Most basic type of hydrocarbon
Carbón bonded to 4 other atoms
General fromula is C n
H
2n+2
End in suffix –ane
Name derived from number of carbons in hydrocarbon chain. KNow the following:
Part of name derived from # carbons
Meth-
Eth-
Prop-
# carbons in hydrocarbon chain
1
2
3
But-
Pent-
Hex-
Hept-
Oct-
Non-
Dec-
4
5
6
7
8
9
10
Write the molecular formula for the following:
1.
Propane___________________
2.
Hexane ____________________
3.
Octane _____________________
4.
Methane _____________________
5.
Butane _______________________
24
Alcohols
Some hydrogen atoms in an alkane chain replaced with hydroxyl -OH
Name is derived from its parent alkane
Name ends with –ol
WHen there is more tan one carbón upon which the hydroxyl group can be present, a number is placed in front of the name to inducate which carbón in the hydrocarbon chain has the –OH group attached
25
26
27
28
29
30