Introduction to Solid State Physics
advertisement

SEVENTH EDITION Introduction to Solid State Physics CHARLES K IT TEL
14 Diamagnetism and Paramagnetism
LANGEVIN DIAMAGNETISM EQUATION
417 QUANTUM THEORY OF DIAMAGNETISM OF MONON UCLEAR SYSTEMS
419 PARAMAGNETISM
420 QUANTUM THEORY OF PARAMAGNETISM
420 Rare earth ions
423 Hund rules
424 Iron group ions
425 Crystal field splitting
426 Quenching of the orbital angular momentum
426 Spectroscopie splitting factor
429 Van Vleck temperature-independent paramagnetism 430 COOLING BY ISENTROPIC DEMAGNETIZATION
Nuclear demagnetization
431 432 PARAMAGNETIC SUSCEPTffiILITY OF COND UCTION ELECTRONS
433 SUMMARY
436 PROBLEMS
436 1. Diamagnetic susceptibility of atomic hydrogen
2. Hund rules
3. Triplet excited states
4. Heat capacity from internaI degrees of freedom
5. Pauli spin susceptibility
6. Conduction electron ferromagnetism
7. Two-Ievel system
8. Paramagnetism of S 1 system
=
REFERENCES
436 437 437 438 438 438 440 440 440
NOTATION : In the problems treated in this chapter the magnetic field B is always
closely equal to the applied field Ba, so that we write B for Ba in most instances.
t
+ -------Or---------~T_--------------------------------
Pauli paramagnetism (metals)
Temperature
Diamagnetism
Figure 1 Characteristic magnetic susceptibilities of diamagnetic and paramagnetic substances.
416
CHAPT ER
14: DIAMAGNE T ISM AND PARAMAGNE TISM
Magnetism is inseparable from quantum m echan ics, for a strictly classical
system in thermal equilibrium can display no magnetic moment, even in a
magnetic field . The magnetic moment of a free atom has three p rincipal
sources: the spin with which electrons are endowed; their orbital angular mo­
mentum about the nucleus; and the change in the orbital moment induced by
an applied magne tic field.
The first two effects give paramagnetic contributions to the magnetization ,
and the third gives a diamagne tic contribution . In the ground Is state of the
h ydrogen atpm the orbital moment is zero , and the magnetic moment is that of
the electron spin along with a small induced diamagne tic moment. In the 1S2
state ofhelium the sp in and orbital moments are both zero , and there is only an
induced moment. Atoms with filled electron shells have zero spin and zero
orbital moment: these moments are associated with unfilled shells.
The magnetization M is defined as the magnetic moment per unit volume.
The magnetic susceptibility p e r unit volume is defined as
M
(Ce S)
x = 13 '
(SI) X = /-LoM
B
(1)
where B is the macroscopic magne tic field intensity. In both systems of units X
is dimensionless. We shall sometimes for convenience refer to MIB as the sus­
ceptibility without specifying the syste m of units .
Quite frequ e ntly a susceptibility is defi ned refe rred to unit mass Or to a
mole of the substance . The molar susceptibility is written as XM ; the magnetic
moment per gram is sometimes writte n as CT. Subs tances with a negative mag­
netic susceptibility are called diamagnetic. Substances with a positive suscepti­
bility are called paramagnetic, as in Fig. 1.
O rdered arrays of magn etic moments are discussed in Chapter 15; the
arrays may be fe rromagne tic, ferrimagnetic, antiferromagne tic, helical, or
more complex in fo rm. N uclear magnetic moments give rise to nuclea r
paramagne tism . Magnetic moments of nuclei are of th e order of 10- 3 times
smalle r than the magnetic momen t of th e electron.
LANGEVIN DIAMAGNETISM EQUATION
Diamagnetism is associated with the tendency of electrical charges par­
tially to shield the in terior of a body from an applied magnetic field. In electro­
magne tism we are fam iliar with Lenz's law: when the fl ux th rough an electrical
circuit is changed, an induced current is set up in such a direction as to oppose
the flux change .
417
418
In a superconductor or in an electron orbit within an atom, the induced
current persists as long as the field is present. The magnetic fie ld of the induced
current is opposite to the applied field, and the magnetic moment associated
with the current is a diamagnetic moment. Even in a normal metal there is a
diamagnetic contribution from the conduction electrons, and this diamag­
netism is not destroyed by collisions of the electrons.
The usual treatment of the diamagnetism of atoms and ions employs the
Larmor theorem : in a magnetic field the motion of the electrons around a
central nucleus is , to the first order in B, the same as a possible motion in the
absence of B except for the superposition of a precession of the electrons with
angular frequency
(ces)
(SI)
w = eB/2mc
w
= eB/2m
.
(2)
If the field is applied slowly, the motion in the rotating reference system will be
the same as the original motion in the rest system before the application of the
field .
If the average electron current around the nucleus is zero initially, the
application of the magnetic field will cause a finite current around the nu­
cleus. The current is equivalent to a magnetic moment opposite to the applied
field. It is assumed that the Larmor frequency (2) is mu ch lower than the fre­
q uency of the original motion in the central field . This condition is not satisfied
in free carrier cyclotron resonance, and the cyclotron frequency is twice the
freq uency (2).
The Larmor precession of Z electrons is equivalent to an electric current
1 = (charge)(revolutions per unit time)
(SI)
= (-
eB)
Ze) ( - 1 . - .
271' 2m
(3)
The magnetic moment I.L of a current loop is give n by the product
(current) X (area of the loop). The are a of the loop of radius p is 7TP'2. We have
(S I)
JI.
=-
ZtfB
4m (p'l) ;
(4)
Here (p'2) = (x'2) + (y'2) is the mean square of the perpendicular distance of the
electron from the field axis thro tigh the nucleus. The mean square distance of
the electrons from the nucleus is (r'2) = (x 2) + (y2) + (Z2). For a spherically
symmetrical distribution of charge we have (x 2) = (y2) = (Z2), so that (r 2) =
i(p2).
From (4) the diamagnetic susceptibility per unit volume is, if N is the
number of atoms per unit volume,
2
= NI.L = _ NZe (r'2)
(ces)
(5)
X
B
6mc'2
'
14
2
x = ILQNIl- =
(SI)
Diamagnetism and Paramagnetism
ILQNZe (r 2 )
B
6m
This is the classical Langevin result.
The problem of calculating the diamagnetic susceptibili ty of an isolated
atom is reduced to the calculation of (r 2 ) for the electron distribution within the
atom . The distribution can be calculated by quantum mechanics .
Experimental values for neutral atoms are most easily obtained for the
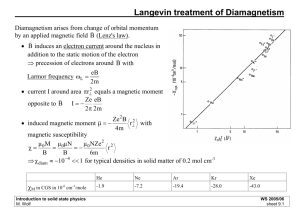
inert gases. Typical experimental values of the molar susceptibilities are the
following :
XM in CGS in 10- 6 cm3lrnole:
He
Ne
Ar
Kr
Xe
-1.9
-7 .2
-19.4
-28.0
-43.0
In dielectric solids the diamagnetic contribution of the ion cores is de­
scribed roughly by the Langevin result. The contribution of con duction elec­
trons is more complicated, as is evident from the de H aas-van Alphen effect
discussed in Chapter 9.
QUANTUM THEORY OF DIAMAGNETISM OF MONONUCLEAR SYSTE MS
From (G . 18) the effect of a magnetic field is to add to the hamiltonian the
terms
ieh
e2
J-C = -(V' . A + A· V') + - A2
2mc
2mc2
,
(6)
for an atomic electron these tenns may usually be treated as a small perturba­
tion . If the magnetic field is uniform and in the z direction , we may write
A x = -~yB ,
hB,
Ay =
Az = 0 ,
(7)
and (6) becomes
iehB(d
J-C = - - x2mc
dy
d)
2
B
2
e - (x 2 + y2)
- y- + 2
dx
8mc
(8)
The first term on the right is proportional to the orbital angular mUlnen­
tum component Lz if r is measured from th e nucleus. In mononuclear syste ms
this term gives rise only to paramagnetism . The second term gives fo r a spheri­
cally symmetric system a contribution
2
E' =
2
e B2 (r 2 )
-1
n1C 2
(9)
'
419
moment is
The
netic:
in
with
ta
is
in:
lar oxygen and organic """<'>,","'0
4. Metals<
The
1l"15"~;U"
moment of an atom or ion in free space is given
where the total angular momentum
angular momenta<
The constant 1'îs the ratio of the
tum; l' is called the
a
g
defined by
g = 2.
the Landé equation
For an
factor is
g
=l +
IiL and
liS
moment to the angular momen­
as
For a free atom the g
~---'----~--'---'-
14
s::
'If
.02
ms
( ///
--',
1.00 1
4
1 0.75
IJ.z
0.
8. 0 .50
2ILB
".
Diamagnetism and Paramagnetism
"s::
- IL
B025
1
-2
e
c....
!J.
o1
o
I
1
i i
1.0
0.5
1.5
2.0
ILBlkBT
Figure 2 Energy level splitting for one electron
in a magnetic field B directed along the positive z
axis. For an electron the magnetic moment JL is
opposite in sign to the spin S, so that JL =
-gJLBS. In th e low energy state the magnetic
moment is paraIJel ta the magnetic field.
Figure 3 Fractional populations of a two-level
system in thermal equilibrium at temperature T
in a magnetic field B. Th e magnetic moment is
proportional ta the difference between the two
curves.
The Bohr magneton J-tB is defined as eh/2mc in ces and eh/2m in SI. It is
closely equal to the spin magnetic moment of a free electron .
The energy levels of the system in a magnetic field are
U
- P' B
=
mjgJ-tBB ,
=
(14)
where mj is the azimuthal quantum number and has the values J, J - l, ... ,
- J. For a single spin with no orbi tal moment we have mj = ± i and g = 2,
whence U = ± J-tBB. This splitting is shown in F ig. 2.
If a system has only two levels the equilibrium populations are, with
T == kBT,
exp(J-tBIT)
NI
(15)
exp(j.LBiT) + exp(- j.LBIT) ,
N
Nz
N
exp(- J-tB IT)
exp(J-tBIT) + exp( - j.LBiT) ,
(16)
here N j , N z are the populations of the lower and upper levels, and
N = N j + N 2 is the total number of atoms. The fractional populations are plot­
ted in Fig. 3.
The projection of the magnetic moment of the upper state along the field
direction is - J-t and of the lower state is J-t. The resultant magnetization for N
atoms per unit volume is , with x == J-tB/kBT,
M = (N I - N 2 )J-t = NJ-t ·
For x
~
l , tanh x
= x,
eX - e- X
x
e
+ _, = NJ-t tanh x .
e
(17)
and we have
M
=NJ-t(J-tB/kBT)
(18)
In a magnetic field an atom with angular momentum quantum number J
has 2J + 1 equally spaced energy levels. The magnetization (Fig. 4) is given by
M = NgJJ-tB Bj(x) ,
(x == gJ J-tBB/k BT ) ,
(19)
421
422
7.00
1TIIInITT':D:o:F:::P:I5F~FiTïi'
BIT in
kG deg- L
Figure 4 Plot of magnetic moment versus BIT for sphe rical samples of (1) potassium ch romium
alum, (II) ferric ammonium alum , and (III) gadolinium sulfate octahydrate. Over 99.5% magnetic
saturation is achieved at 1.3 K and about 50,000 gauss. (ST). After W. E . Henry.
where the Brillouin function BI is defined by
B,(x)
.
=
2J + 1 ctnh ((2J +
2J
2J
l)x)
- - 1 ctnh ( - x )
2J
2J
Equation (17) is a special case of (20) for J = t.
For x <s:; l, we have
1
x
x3
ctnh x = - + - - +
x
3
45
(20)
(21)
and the susceptibility is
M
-=
B
NJ(J
+
1)g2JL~
C
3k B T
T
(22)
H ere p is the effective number of Bohr magnetons, defined as
p
== gU(J + 1)F /2
.
(23)
14 Dianwgnetism and Paranwgnetism
40~--------~----'~~-~------~~
s
i
Temperature, Je
Figure 5 Plot of l/X vs T for a gadolinium salt, Gd(C zH 5 S0 4h
Onnes,)
Curie law, (Aftel' L. C. Jackson and
Rare Earth Ions
.
straight line
the
Even in the
no other
atom
state is charac­
maximum
maximum value of the
of S,
S allowed
momentum
exclusion
consistent with
to IL - SI when the
shell is more than half fulL
ruIe
L
0, so
is
different
14
Table l
Diamagnetism and Paramagnetism
Effective magneton numbers p for trivalent lanthanide group ions
(Near room tempe rature)
Ion
Configuration
c é+
Pr 3 +
4P5s2 p6
4j25s 2 p6
4P5s 2 p6
4f 4 5s2 p6
4f s5s 2p 6
4f6 5s 2 p6
4F5s 2 p6
4j'B5s 2 p6
4f 9 5s 2 p6
4po5s2 p6
4f1l5 s2 p6
4P 25s 2 p6
4P 3 5s 2 p6
Basic level
---­
p(calc) =
gU(] + 1)]JJ2
p(exp),
approximate
__.=:l
Nd 3 +
Pm 3 +
Sm 3 +
Eu3+
Gd 3 +
Tb 3 +
D y 3+
Ho3 +
E r3+
Tm 3 +
Yb 3 +
2F
s I2
3H
4
41 912
514
6H
sf2
7F
o
8S 712
7F
6
6H
1SI2
s Is
41 1S12
3H
6
2F7i2
2. 54
3. 58
3.62
2. 68
0.84
0
7.94
9.72
10.63
10.60
9.59
7. 57
4.54
2.4
3. 5
3.5
1.5
3.4
8.0
9.5
10.6
10.4
9.5
7.3
4.5
The second Hund rule is best approached by model calculations. Pauling
and Wilson, l for example, give a calculation of the spectral terms that arise fro m
the configuration p2. The third Hund rule is a consequence of the sign of the
spin-orbit interaction: For a single electron the energy is lowest when the spin
is antiparallel to the orbital angular momentum. But the Iow energy pairs mL,
ms are progressively used up as we add electrons to the shell; by the exclusion
principle when the shell is more th an half full the state of lowest energy neces­
sarily has the spin parallel ta the orbit.
Consider two examples of the Hund fuIes : The ion c é+ has a single f
electron; an f electron has l = 3 and s = i. Because the f shell is less than half
full, the ] value by the preceding rule is IL - SI = L - ! = l The ion Pr 3 + has
two f electrons: one of the mIes tells us that the spins add to give S = 1. Both f
electrons cannot have ml = 3 without violating the Pauli exclusion principle, so
that the maximum L consistent with the Pauli principle is not 6, but 5. The]
value is IL - si = 5 - 1 = 4.
Iron Group Ions
Table 2 shows that ~he experimental magneton numbers for salts of the iron
transition group of the p elt'iodic table are in poor agreement with (18). The
values often agree quite weil with magneton numbers p = 2[S(S + 1)]112 calcuIL. Pauling and E . B. Wilson, Introduction to quantum mechanics, McGraw-Hill, 1935,
pp. 239-246.
425
426
Table 2
E ffective magneton numbers for iron group ions
Ion
Configuration
Ti3+, y4+
y 3+
Cr3+, y2+
M n 3+, Cr+
F e 3+, Mn 2 +
Fe 2+
C o 2+
Ni 2+
Cu 2 +
3d l
3d 2
3d 3
3d 4
3d 5
3d6
3d7
3d 8
3d 9
Basic
level
2D
3F
3I2
2
4F 3/2
5DO
65
51 2
5D
4
4F 9/2
3F
4
2D5/ 2
=
p(calc) =
gU(] + 1)]112
2[$($ + 1)]112
p(exp)a 1.55
1.63
0. 77
0
5.92
6.70
6.63
5.59
3.55
1. 73
2.83
3.87
4.90
5.92
4.90
3.87
2.83
1.73
1.8
2.8
3.8
4.9
5.9
5.4
4.8
3.2
1.9
p(calc)
"Representative values.
lated as if the orbital moment were not there at ail. We say that the orbital
moments are quenched.
Crystal Field Splitting
The difference in behavior of the rare earth and the iron group salts is that
the 4f shell responsible for paramagnetism in the rare earth ions lies deep
inside the ions, within the 5s and 5p sheIls, whereas in the iron group ions the
3d shell responsible for paramagnetism is the outermost shell. The 3d shell
experiences the intense inhomogeneous electric field produced by neighboring
ions. This inhomogeneous electric field is called the crystal field. The interac­
tion of the paramagnetic ions with the crystal field has two major effects: the
coupling of L and S vectors is largely broken up, so that the states are nO longer
specified by their J values; further, the 2L + l sub levels belonging to a given L
which are degenerate in the fre e ion may nOw be split by the crystal field , as in
Fig. 6. This splitting diminishes the contribution of the orbital motion to the
magnetic moment.
Quenching of the Orbital Angular Momentum
In an electric field directed toward a fixed nucleus, the plane of a classical
orbit is fixed in space, so that aIl the orbital angular momentum components Lx>
Ly, Lz are constant. In quantum theory one angular momentum component,
usually taken as Lz, and the square of the total orbital angular momentum L2 are
constant in a central field. In a noncentral field the plane of the orbit will move
about; the angular momentum components are no longer constant and may
average to zero. In a crystal Lz will no longer be a constant of the motion,
although to a good approximation L2 may continue to be constant. When Lz
averages to zero, the orbital angular momentum is said to be quenched. The
14 Diamagnetism and Paramagnetism
@
@
@
===== P"Py
y
y
@
®
®
(a)
(b)
(c)
-
---pz
(d)
Figure 6 Consider an atom with orbital angular momentum L = l placed in the uniaxial crystalline
electric field of the two positive ions along the z axis. In the free atom the states mL = ± l, 0 have
identical energies-they are degenerate. In the crystal the atom has a lower energy when the
electron cloud is close to positive ions as in (a) th an when it is oriented midway between them, as
in (b) and (c). The wavefunctions that give rise to these charge densities are of the form zf(r), xf(r)
and yf(r) and are called the Pz, Px, Py orbitaIs, respectively. In an axially symmetric field, as shown,
the Px and Py orbitaIs are degenerate. The energy levels referred to the free atom (dotted !ine) are
shown in (d). If the electric field does not have axial symmetry, ail three states will have different
energies.
magne tic moment of astate is given by the average value of the magnetic
moment operator I-tB(L + 2S). In a magnetic field along the z direction the
orbital contribution to the magnetic moment is proportion al to the quantum
expectation value of L z; the orbital magnetic moment is quenched if the me­
chanical moment Lz is quenched.
When the spin-orbit interaction energy is introduced, the spin may drag
sorne orbital moment along with it. If the sign of the interaction favors paraUel
orientation of the spin and orbital magnetic moments, the total magnetic mo­
ment will be larger than for the spin alone, and the g value will be larger than 2.
The experimental results are in agreement with the known variation of sign of
the spin-orbit interaction: g > 2 when the 3d shell is more than half full, g = 2
when the shell is half full , and g < 2 when the shell is less than half full .
We consider a single electron wi th orbital quantum number L = 1 moving
about a nucleus, the whole being placed in an inhomogeneous crystalline elec­
tric field. We omit electron spin.
In a crystal of orthorhombic sym metry the charges on neighboring ions
will produce an electrostatic potential cp about the nucleus of thJ form
ecp = AX2
+ B y2 - (A + B )Z2 ,
(24)
where A and B are constants. This expression is the lowest degree polynomial
in x, y, z which is a solution of the Laplace equation V2 cp = 0 and compatible
with the symmetry of the crystal.
427
428
Uz = zf(r)
Uy = yf(r) ;
are normalized.
=
2Ui
,
=
0 .
Consider
dx dy dz ;
(28)
the integral
the diagonal matrix
elements:
dx dy dz
+
(29)
where
dx
The
dz ;
their angular lobes
o.
This effect is
momentum,
age is zero in
magnetic moment also
ParamilgnetÎttm
(30)
- À/à l
the
,
hetween
g
g
'See L. Orgel, Introduction to transition
references are given by
D. Sturge, Phys.
1966; extensive
430
Van Vleck Temperature-Independent Paramagnetism
We conside r an atomic or molecular system which has no magnetic mo­
ment in the ground state, by which we mean that the diagonal matrix element
of the magnetic moment operator J.Lz is zero.
Suppose that there is a nondiagonal matrix element (slJ.LzIO) of the magnetic
moment operator, connecting the ground state with the excited state s of
energy  = Es - Eo above the ground state. Then by standard perturbation
theory the wavefunction of the ground state in a weak field (J.LzB ~ Â) becomes
°
(32)
and the wavefunction of the excited state becomes
(33)
The perturbed ground state now has a moment
(34)
and the upper state has a moment
(35)
There are two interesting cases to consider:
Case (a). Â ~ kBT. The surplus population in the ground state over the
excited state is app roximately equal to NÂ/2kB T, so that the resultant magneti­
zation is
M = 2BI(slJ.LzIO)1
2
NÂ
2k B T '
Â
(36)
which gives for the susceptibility
(37)
Here N is the number of molecules per unit volume. This contribution is of the
usuaI Curie form , although the mechanism of magnetization here is by polariza­
tion of the states of the system, whereas with free spins the mechanism of
magnetization is the redistribution of ions among the spin states. We note that
the splitting  does not enter in (37).
Case (h) . Â ;? kB T . Here the population is nearly aIl in the ground state, so
that
M = 2NBI(slJ.LzIO>1
Â
2
(38)
The susceptibility is
(39)
Diamagnetism
type of contribution
Paramagnetism
known as Van Vleck
COOLING DY
The first metbc,d
the
partly lined
is also lowered if
1) .
in
3The method was suggested by P Debye, Ann.
Am, Chem, Soc, 49, 1864 (1927). For many purposes
dilution
which operates
He' play the raIe of atoms in a gas, and
12.
Giauque,
by the
solution in
SUI)pla'ntt~d
431
432
Total
Spin
Spin
Time-
Lattice
Before
1
TimeBe ore
New equili brium
Time at which
magnetic field
is removed
:\cw equilibrium
Time at which magnetic field is l'emoved Figure 7 During adiabatic dem agnetization the total entropy of the specimen is constant. For
effective cooli ng the initi al entropy of the lattice should be small in comparison with the entropy of
the spi n sys tem.
The steps carried out in the cooling process are shown in F ig. 8. The field
is applied at temperature T l with the specimen in good thermal contact with
the surroundings, giving the isothermal path ab. The specimen is then insu­
lated (!la- = 0) and the fi eld removed; the specimen follows the constant en­
tropy path he, ending up at temperature T 2 . The thermal contact at Tl is pro­
vided by helium gas, and the thermal contact is broken by removing the gas
with a pump.
Nuclear Demagnetization
The population of a magne tic sublevel is a function only of f.LB l kBT , hence
of BIT. The spin -system entropy is a function only of the population distribu­
tion ; hence the spin entropy is a function only of BIT. If Bt>. is the effective field
that corresponds to the local interactions, the final temperature T 2 reached in
an adiabatic demagnetization experiment is
1
T 2 = Tl (Bt>.IB ) ,
1
(41)
whe re B is the initial field and Tl the initial temperature.
Because nuclear magne tic moments are weak, nuclear magnetic interac­
tions are much weaker than similar electronic interactions. We expect to reach
a tem pe rature 100 times lower with a nuclear paramagnet than with an electron
paramagnet. The initial temperature Tl of the nuclear stage in a nuclear spin­
cooling experiment must be lower than in an electron spin-cooling experiment.
If we start at B = 50 kG and Tl = 0.01 K, then f.LBlkBT l = 0.5, and the e n­
14
Diamagrwtism and Paramagfletism
0.7,r---------------------------------------------------------~
0.6
~
~
~ 0.5
§
S ~4
B = 0; BA = 100 gauss
~
g 0. 3
~
~
S Qi
~
0.1
o6
L
~
10
15
do
~5' j'J
T, mK
·' igure 8 Entropy for a , pin 1 sys tem as a fun etion of te mpera ture, assumin g an intern aI random
magne tic field Be:. of 100 gauss. The specimen is magnetized iso th e rmall y along ab , and is th en
insulated thermally. The external magnetie field is turned off al ong be. In ord e r to keep the figure
on a reasonable seale the initial tem pe rature Tl is lower th an wo uId be us ed in practice, and so is
the exte rn al magnetic fi eld .
tropy decrease on magnetization is ove r 10 percent of the maxim um spin en­
tropy . This is sufficient to overwhelm the lattice and from (4 1) we estimate a
final te mperature T 2 = 10- 7 K. The first 4 nuclear cooling experiment was car­
ried out on Cu nudei in the metal, starting from a fi rst stage at about 0.02 K
as attained by electronic cooling. The lowest tempe rature reached was
1.2 x 10- 6 K.
The results in Fig. 9 fit a line of the fonn of(41) : T z = T 1(3.1 /B) with B in
gauss, so that B11 = 3.1 gauss. This is the effective interaction field of the mag­
netic moments of the Cu nuclei. The motivation for using nud ei in a metal is
that conduction electrons help e nsure rapid thermal contact of lattice and nu­
dei at the tempe rature of the first stage . The present record5 for a spin temper­
ature is 280 pK, in rhodium.
PARAMAGNETIC SUSCEPTIBILITY OF CONDUCTION ELECTRONS
We are going to try to show how on the basis of these stati sti cs the fa ct th at many
me tals are diamagnetic, or only weakl y paramagnetic, can be brought into agree­
me nt with tb e existence of a magnetic mom e nt of tbe e lectrons .
W. Pauli, 1927
Classical fr ee electron theory gives an un satisfactory account of the para­
magnetic susceptibility of the conduction electrons. An electron has associated
with it a magne tic mom ent of one Bohr magneton , /-La. One might expect that
4N . Kurti , F . N . H . Robinson, F. E. Simon, and D . A. Spohr, Nature 178 , 450 (1 956); for
reviews see N. · Kurti, Cryogenies 1, 2 (1960); Adv. in Cryogenie Engineering 8, 1 (1963).
sp. J. Hakonen e t al ., Ph ys . Rev. Lett. 70, 2818 (1993).
433
434
Initial magnetic field in kG
lonr---T5--------~lrO--------~20~---3TO~
9
8
7
1
6
~
5
e
4
.,1;0
u
Ë
.S
3
lL-__L-~~~~~~--------~--~
0.3 0.6
2
Initial BIT in 106 G/K Figure 9 Nuclear demagnetizations of copper nuclei in the metal, starting from 0.012 K and
various fields . (After M. V. Hobden and N. KurtL)
the conduction electrons wo uld make a Curie-type paramagnetic contribution
(22) to the magnetization of the metal: M = N/-L~BlkB T. Instead it is observed
that the magnetization of most normal nonferromagnetic metals is independent
of temperature.
Pauli showed that the application of the Fermi-Dirac distribution (Chap­
ter 6) w6uld correct the theory as required. We firs t give a qualitative explana­
tion of the situation . The result (18) tells us that the probability an atom will be
lined up parallel to the field B exceeds the probability of the antiparallel orien­
tation by roughly /-LBlkB T. For N atoms per unit volume, this gives a net mag­
netization = N/-L2Blk BT, the standard result .
Most conduction electrons in a metal, however, have no possibility of
turning over when a fiel d is applied, because most orbitais in the Fermi sea
with parallel spin are already occupied. O nly the electrons within a range kBT
of the top of the Fermi distribution have a chance to turn over in the field; thus
only the fraction TIT F of the total number of electrons contribute to the suscep­
tibility. Hence
N/-L2B T
N/-L2
M =--' -=--B
kBT
TF
kBTF
which is independent of temperature and of the observed order of magnitude.
We now calculate the expression for the paramagnetic susceptibility of a
free electron gas at T ~ TF. We follow the method of calculation suggested by
Fig. 10. An alternate derivation is the subject of Problem 5.
14 Diamagnetism and Paramagnetism
Total energy, kinetic +
magne tic, of electrons
l
~
1
Parallel
ta field
Dffi~~~
o~~
,
<
~
....
1
Density of
orbitais
1
(b)
(a)
Figure 10 Pauli paramagnetism at absolu te zero; the orbitais in the shaded regions in (a) are
occupied . The numbers of electrons in the " up " and "down" band will adjust ta make the energies
equal at the Fermi level. The chemical potential (Fermi level) of the moment up electrons is equal
to that of the moment down electrons. In (b) we show the excess of moment up electrons in the
magnetic field.
The concentration of electrons with magnetic moments parallel to the
magnetic field is
N+ = -
l
2
J
'F
- l'-B
dE D (E
+
fJ-B )
== -l
2
written for absolute zero. Here ~D(
E
:2
l
EF
dE D(E)
+ -l
2
0
+
fJ-B D (EF) , fJ-B ) is the densitv• of orbitaIs of one
spin orientation, with allowan ce fo r the downward shift of energy by - fJ-B .
The approximation is written for kBT <{ EF •
The concentration of electrons with magnetic moments antiparallei to the
magnetic field is
N_ = - l
JEF dE D(E - fJ-B) == -l
21'-B
l'FdE D (E) -
20
-l fJ-B D(EF) 2
The magnetization is given by M = fJ-(N + - N _), so that
3N fJ-2
M = fJ-2 D (EF) B = - k -B
2 BTF
(42)
with D(EF) = 3N/2EF = 3N/2k B T F fro m C hapter 6. The result (42) gives the
Pauli spin magnetization of the conduction electrons, for kBT <{ EF •
In deriving the paramagnetic susceptibility, we have supposed that the
spatial motion of the electrons is not affected by the magnetic field. But the
wavefunctions are modified b y the magnetic fie ld; Landau has shown that for
435
436
(43)
B.
the
by
UUU1H.l<U.'y
Ipl"~r"n,,,
of atomic
atomic
The
high for transition
heat
Z is X
(Langevin)
the maximum
S
consistent with this S. The
and IL - S if the shell is Jess
.. .. is
14
80
tS
r
iT
T
T
1
1
Diamagnetism and Paramagnetism
1
IIT ­
7.0
6.0
5.0
~
~
\
\ -r--r-2_
E
"
~
\
w
1
8
"__ g
Cr
4.0
~
0.
'"
:l"
V>
~
3.0
2.0
--~_ I ~
\
//
~~/
... V
.......-~w '\
- r--_ "­
f--
I r- - -
_J-_+-_r-zr- v
_
1'1
1.0
- -~-
~Nb
-
Rh
l
~
Na K--
'"
1R'b"'f--- T--t--
-+--1f--+_-J-::H::lr r--- 0
0
200
400
600
J
800
J
1000 1200
T. in K
1400
-
Ta
J
1600
1800
2000
2200
Figure 11 Temperature dependence of the magnetic susceptibility of metals. (Courtesy of C.
Kriessman .)
J.
2. Huml mles. Apply the H und rules to find the ground state (the basic level in the
notation ofTable 1) of (a) E u + +, in the configuration 4[1 5S 2p6; (b) Yb3+ ; (c) Tb 3 + . The
results fo r (b) and (c) are in Table 1, but you should give the separate steps in applyi ng
the rules.
3. Triplet excited states. Some organic molecules have a triplet (S = 1) excited state at
an energy kBil above a singlet (S = 0) ground state. (a) Find an expression for the
magnetic moment (J-L ) in a fie ld B. (b) Show that the susceptibility for T p il is
approximately independent of il. (c) With the help of a diagram of energy levels
versus field and a rou gh sketch of entropy versus field , explain how this system might
be cooled by adiabatic magne tiza tion (not demagnetization).
437
438
4. Consider two-Ievel system with
and Iower states; the splitting may arise from
Show that the hoat capacity per system is
c
capacity
interaction between nuclear and electronic electron spin order) '1'l'",L111!,' are often detected experi­ in the heat capacity in the region T P À. interaetions (see
with
fields al 50 spin
of a conduction eleetron gas at abso­
another method.
be the eoneentrations
eleetrons.
Show that in a magnetie
field B the total energy of the spin-up band in a free eleetron gas is
+(),
where
similar
for the
6. in zero magnetic field. Find a
+ E - with respect to , and solve
value of , in the approximation , ~ 1. Go
in agreement with
to show that the
approximate the eHeet of
inter­
assume that eleetrons with parallel
aetions among the eonduction electrons if
is positive, while electrons with
with each other \vith energy
of Problem 5
not interact with each other.
Show with the
(1
+ () ;
find a similar expression for
the total energy and
limit {, ~ 1. Show that the magnetization is
for
in the
so
the
interaction enhances the susceptibility. (c) Show that with B = 0
the total energy is unstable at' 0 when V >
this is satisfied a
paramagnetic state. Because of
neUc state ({, "'" 0) will have a lower energy th an
the assumption t: ~ l, this is a sufficient condition for
but it may
not be a neccssary condition. It is known
14 Dinmaf!:netism and Paramagnetism
0.5
--,------r---,---,---,--i
r-j
.°l
""
.~
1
Level2
j
Level l
,:;
0.3
"
S
è
,J
'"p,
8
0.2
0.1
00
4
5
6
x = Tlt.
Figure 12 Heat capacity of a two-level system as a function of T/t;,., where t;,. is the level splitting.
The Schottky anomaly is a very useful tool for determining energy level splittings of ions in rare­
earth and transition-group metals, compounds, and alloys.
0.008
;.0
0006
eNT" = 4.3 x
1
1
(3
E
E
0.004
.S
h
"
u
0.002
0.002
0.004
0006
0008
0.01
TO, in KJ
Figure 13 The normal-state heat capacity of gallium at T < 0.21 K. The nuclear quadrupole
(G ct: T 2) and conduction electron (G 0: T) contributions dominate the heat capacity at very low
temperatures. (After
K Phillips.)
439
7. Two-level system. The result of Problem 4 is often seen in another form.
If the two
energy levels are at à and -il,
that the energy and heat capacity are
u=
c=
of à are
proportional to the tem­
to the heat capacity of dilute
1519
It is al50 used in the
8. Itystem.
Find the magnetization as a function
field and temperature for a system of spins with S 1, moment
n. (b) Show that in the li mit li-B <{ kT
result is
­
A. Abragam and B. Bleaney. Electron
resonance
tom, Dover, 1986.
B. G. Casimir, Magnetism and very
tempe ratu res, DoveT, 1961. A c1assic. Darby and K.
R. Taylor, Physics of rare earth
Halsted, 1972. A. J. Freeman, The actinides: electronic structure and related properties, Academie, 1974.
R. D. Hudson. Princip les and
Elsevier, 1972.
North-Holland, 1970.
Knoepfel, Pu/sed
Lounasmaa,
and methods below 1 K, Academie Press, 1974.
Introduction ta transition metal
2nd ed., Wiley, 1966.
Van Vleck, The theory
Oxford, 1932.
deriva­
tions of basic theorems.
G. K. White, 3rd
Oxford, 1987.
R.
White, Quantum theory
A. J. Freeman and G. H. Lander,
actinides. NorthHolland, 1984-1993.
91 (1967).
Sturge, "Jahn-Teller effect in solids," Solid state
O'Brien and C. C. Chancey, "The
effect: An introduction and current re­
(1993),
view," Amer. J, Physics 61,