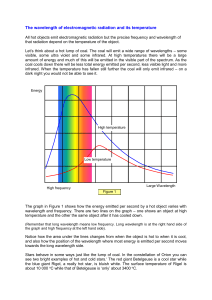
From lowest energy to highest energy, which of the following
advertisement

From lowest energy to highest energy, which of the following correctly orders the different categories of electromagnetic radiation? A) infrared, visible light, ultraviolet, X rays, gamma rays, radio. B) visible light, infrared, X rays, ultraviolet, gamma rays, radio. C) radio, X rays, visible light, ultraviolet, infrared, gamma rays. D) gamma rays, X rays, visible light, ultraviolet, infrared, radio. E) radio, infrared, visible light, ultraviolet, X rays, gamma rays. From lowest energy to highest energy, which of the following correctly orders the different categories of electromagnetic radiation? A) infrared, visible light, ultraviolet, X rays, gamma rays, radio. B) visible light, infrared, X rays, ultraviolet, gamma rays, radio. C) radio, X rays, visible light, ultraviolet, infrared, gamma rays. D) gamma rays, X rays, visible light, ultraviolet, infrared, radio. E) radio, infrared, visible light, ultraviolet, X rays, gamma rays. The frequency of a wave is The frequency of a wave is A) the number of peaks passing by any point each second. B) measured in hertz (Hz). C) measured in cycles per second. D) equal to the speed of the wave divided by the wavelength of the wave. E) all of the above. A) the number of peaks passing by any point each second. B) measured in hertz (Hz). C) measured in cycles per second. D) equal to the speed of the wave divided by the wavelength of the wave. E) all of the above. We can see each other in the classroom now because we A) emit infrared light. B) emit thermal radiation. C) reflect visible light. D) reflect infrared light. E) emit visible light. We can see each other in the classroom now because we A) emit infrared light. B) emit thermal radiation. C) reflect visible light. D) reflect infrared light. E) emit visible light. 1 How are wavelength, frequency, and energy related for photons of light? How are wavelength, frequency, and energy related for photons of light? A) Longer wavelength means higher frequency and lower energy. B) Longer wavelength means lower frequency and higher energy. C) There is no simple relationship because different photons travel at different speeds. D) Longer wavelength means higher frequency and higher energy. E) Longer wavelength means lower frequency and lower energy. Learning from from Light Learning Light A) Longer wavelength means higher frequency and lower energy. B) Longer wavelength means lower frequency and higher energy. C) There is no simple relationship because different photons travel at different speeds. D) Longer wavelength means higher frequency and higher energy. E) Longer wavelength means lower frequency and lower energy. Learning goals • • • • Radio Optical What types of light spectra can we observe? What types of light spectra can we observe? How does light tell us about composition? How does light tell us about temperatures? How does light tell us the speed of an object? X-ray Example: Solar Spectrum 2 How does light tell us what things are made of? Hydrogen atom Distinct energy levels lead to distinct emission or absorption lines • Electrons in atoms have distinct energy levels. • Each chemical element, ion, molecule, has a unique set of energy levels. Light contains chemical fingerprints Thought Question Which letter(s) labels absorption lines? • Every atom, ion, and molecule has a unique spectral “fingerprint.” • We can identify the chemicals in gas by their fingerprints in the spectrum. • With additional physics, we can figure out the chemicals’ abundances, densities, temperatures, and more. A B C D E C D E Thought Question Which letter(s) labels the peak (greatest intensity) of infrared light? Thought Question Which letter(s) labels absorption lines? A B A B C D E 3 Thought Question Which letter(s) labels the peak (greatest intensity) of infrared light? A B C D E Thought Question Which letter(s) labels emission lines? Thought Question Which letter(s) labels emission lines? A B C D E How does light tell us the temperatures of planets and stars? Thermal Radiation A B C D E Two Properties of Thermal Radiation 1. Hotter objects emit more light at all frequencies per unit area. 2. Hotter objects emit photons with a higher average energy. • Nearly all large or dense objects emit thermal radiation, including stars, planets, you… • An object’s thermal radiation spectrum depends on only one property: its temperature. Thought Question Which is hotter? a) A blue star. b) A red star. c) A planet that emits only infrared light. 4 Thought Question Which is hotter? a) A blue star. b) A red star. c) A planet that emits only infrared light. Thought Question Why don’t we glow in the dark? a) People do not emit any kind of light. b) People only emit light that is invisible to our eyes. c) People are too small to emit enough light for us to see. d) People do not contain enough radioactive material. Doppler shift tells us ONLY about the part of an object’s motion toward or away from us! Thought Question Why don’t we glow in the dark? a) People do not emit any kind of light. b) People only emit light that is invisible to our eyes. c) People are too small to emit enough light for us to see. d) People do not contain enough radioactive material. How does light tell us the speed of a distant object? The Doppler Effect The amount of blue or red shift tells us an object’s speed toward or away from us. Thought Question I measure a line in the lab at 500.7 nm. The same line in a star has wavelength 502.8 nm. What can I say about this star? a) It is moving away from me. b) It is moving towards me. c) It has unusually long spectral lines. 5 Doppler Effect Summary Thought Question I measure a line in the lab at 500.7 nm. The same line in a star has wavelength 502.8 nm. What can I say about this star? a) It is moving away from me. b) It is moving towards me. c) It has unusually long spectral lines. Motion toward or away from an observer causes a shift in the observed wavelength of light: • blueshift (shorter wavelength) ⇒ motion toward you • redshift (longer wavelength) ⇒ motion away from you • greater shift ⇒ greater speed 6